Combined Effect of DNase I and Proteinase K on Dual-Species Biofilm of Campylobacter jejuni and Acinetobacter baumannii
- PMID: 41346417
- PMCID: PMC12673173
- DOI: 10.1155/ijfo/6948459
Combined Effect of DNase I and Proteinase K on Dual-Species Biofilm of Campylobacter jejuni and Acinetobacter baumannii
Abstract
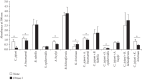
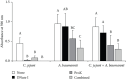
In food-associated environments, foodborne bacteria can form biofilms that are likely to exist as multiple species and are potentially a food safety concern. In this study, we focused on the effects of DNase I and proteinase K on dual-species biofilms containing Campylobacter jejuni and bacterial isolates from food-associated environments. The viable cell counts of C. jejuni differed significantly depending on the counterpart strain in the biofilms. In mature monospecies biofilms, both C. jejuni and Acinetobacter baumannii were susceptible to both enzymes. Acinetobacter baylyi was susceptible only to DNase I, while Staphylococcus epidermidis was susceptible only to proteinase K. Analysis of confocal laser scanning microscopy images of A. baumannii biofilm showed that the protein distribution was consistent with that of the biofilm-embedded cells, whereas it was distinct from the polysaccharide distribution. Among the dual-species biofilms, that of C. jejuni and A. baumannii was the only biofilm susceptible to both enzymes. Combined treatment using DNase I followed by proteinase K was far more effective than DNase I monotherapy against both A. baumannii mono- and dual-species biofilms. Our study suggests that proteins could be a primary target for inactivating biofilm-embedded cells in A. baumannii, and the use of multiple enzymes could be an efficient strategy for biofilm removal.
Keywords: Campylobacter; DNase I; biofilm; dual-species; proteinase K; reduction.
Copyright © 2025 Joo Young Lee and Joo-Sung Kim. International Journal of Food Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Kirk M. D., Pires S. M., Black R. E., Caipo M., Crump J. A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C. L., Hald T., Hall A. J., Keddy K. H., Lake R. J., Lanata C. F., Torgerson P. R., Havelaar A. H., and Angulo F. J., World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis, PLoS Medicine. (2015) 12, no. 12, e1001921, 10.1371/journal.pmed.1001921, 2-s2.0-84953286299, 26633831. - DOI - PMC - PubMed
-
- U.S. Department of Agriculture (USDA), The Potential Health Risk of Acinetobacter baumannii as a Foodborne Pathogen and Attenuating Its Antibiotic Resistance Using Plant-Derived Antimicrobials, 2023, National Agricultural Library. Accessed 12 March 2025, https://www.nal.usda.gov/research-tools/food-safety-research-projects/po....
