Branched‑chain amino acid metabolism and bone metabolism: Implications for osteoporosis pathogenesis and therapeutic strategies (Review)
- PMID: 41347804
- PMCID: PMC12695155
- DOI: 10.3892/ijmm.2025.5706
Branched‑chain amino acid metabolism and bone metabolism: Implications for osteoporosis pathogenesis and therapeutic strategies (Review)
Abstract
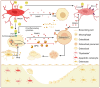
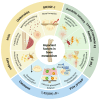
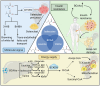
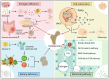
Branched‑chain amino acids (BCAAs) are biologically active amino acids with branched carbon chains, recognized for their diverse biological functions and therapeutic potential. BCAAs have demonstrated promising effects in the prevention and treatment of various conditions, including muscle growth disorders, cardiovascular diseases and cancer. Despite extensive research confirming their targeted therapeutic effects in multiple domains, the mechanisms of action and therapeutic range of BCAAs remain incompletely understood. Osteoporosis, a metabolic bone disease, is a global public health issue characterized by an imbalance between osteoblast‑mediated bone formation and osteoclast‑induced bone resorption, resulting in fragile bones and an elevated risk of fractures. Given the well‑documented therapeutic roles of BCAAs, their potential link to osteoporosis has been explored, emphasizing the influence of BCAA metabolism on bone metabolism. The present review aims to summarize findings on the relationship between BCAA metabolism and osteoporosis, and to investigate the mechanisms by which BCAA metabolism may exert anti‑osteoporotic effects. The review first outlines the fundamental processes and key factors influencing bone metabolism, BCAA metabolism and osteoporosis. It then examines the interactions between these processes and the effects of BCAA metabolism on bone health. Finally, it explores the potential of targeting BCAA metabolic pathways as a future therapeutic strategy for osteoporosis, highlighting BCAAs as a promising target for treating this condition.
Keywords: BCAA metabolism; BCAAs; bone metabolism; osteoporosis; therapeutic target.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
