Autoantibodies neutralizing type I IFNs in a fatal case of H5N1 avian influenza
- PMID: 41348320
- PMCID: PMC12679982
- DOI: 10.1084/jem.20251962
Autoantibodies neutralizing type I IFNs in a fatal case of H5N1 avian influenza
Abstract
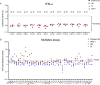
Avian influenza A virus (IAV) H5N1 is an emerging threat of human pandemic. We describe a 71-year-old man who died of H5N1 pneumonia in Louisiana and whose blood contained autoantibodies neutralizing type I IFNs (AAN-I-IFNs), including the 12 IFN-α subtypes (1-10 ng/ml) and IFN-ω (100 pg/ml). Causality between these AAN-I-IFN and lethal outcome of avian influenza in this patient is based on (1) our previous report that AA-I-IFN underlie about 5% of cases of critical pneumonia triggered by seasonal influenza viruses in three cohorts, (2) the rarity of this combination of AAN-I-FNs in individuals over 70 years old (<1%), and (3) the rarity of lethal avian influenza among infected individuals (<1%). AAN-I-IFNs underlie a growing number of severe viral diseases, from arboviral encephalitis to viral pneumonia, particularly in the elderly. This case suggests they can also underlie life-threatening avian H5N1 influenza. The presence of AAN-I-IFN may facilitate infection, replication, and adaptation of zoonotic IAVs to humans and, therefore, human-to-human transmission.
© 2025 Zhang et al.
Conflict of interest statement
Disclosures: J. Eaton reported personal fees from Vantive outside the submitted work. J.A. Vanchiere reported other from Merck, GSK, Pfizer, Enanta, BioCryst, and Innoviva outside the submitted work. J.-L. Casanova reported a patent to PCT/US2021/042741, pending. No other disclosures were reported.
Figures



References
-
- Al Qureshah, F., Le Pen J., de Weerd N.A., Moncada-Velez M., Materna M., Lin D.C., Milisavljevic B., Vianna F., Bizien L., Lorenzo L., et al. 2025. A common form of dominant human IFNAR1 deficiency impairs IFN-alpha and -omega but not IFN-beta-dependent immunity. J. Exp. Med. 222:e20241413. 10.1084/jem.20241413 - DOI - PMC - PubMed
-
- Alotaibi, F., Alharbi N.K., Rosen L.B., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Jeraisy M., Mandourah Y., AlJohani S., Al Harbi S., et al. 2023. Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial. Influenza Other Respir. Viruses. 17:e13116. 10.1111/irv.13116 - DOI - PMC - PubMed
-
- Aydillo, T., Rombauts A., Stadlbauer D., Aslam S., Abelenda-Alonso G., Escalera A., Amanat F., Jiang K., Krammer F., Carratala J., and García-Sastre A.. 2021. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 12:3781. 10.1038/s41467-021-23977-1 - DOI - PMC - PubMed
-
- Bastard, P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.-H., Eto S., Garcia-Prat M., et al. 2021a. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 6:eabl4340. 10.1126/sciimmunol.abl4340 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- St. Giles Foundation
- Rockefeller University
- HHMI/Howard Hughes Medical Institute/United States
- Institut National de la Santé et de la Recherche Médicale
- Imagine Institute
- Paris Cité University
- RR/NCRR NIH HHS/United States
- UL1TR001866/NH/NIH HHS/United States
- R01AI163029/NH/NIH HHS/United States
- COVID-1026207/American Lung Association
- Stavros Niarchos Foundation
- Square Foundation
- Grandir - Fonds de solidarité pour l'enfance
- Fondation du Souffle
- SCOR Corporate Foundation for Science
- Battersea and Bowery Advisory Group
- ANR-10-IAHU-01/French National Research Agency
- ANR-10-LABX-62-IBEID/French National Research Agency
- ANR-20-CE93-003/French National Research Agency
- ANR-21-LIBA-0002/French National Research Agency
- ANR-22-CE15-0046/French National Research Agency
- ANR-22-CE92-0004/French National Research Agency
- ANR-21-RHUS-08/French National Research Agency
- 101057100/HORIZON-HLTH-2021-DISEASE-04
- 824110/European Union's Horizon 2020
- General Atlantic Foundation
- French Ministry of Higher Education, Research, and Innovation
- REACTing-INSERM
- EA20170638020/French Foundation for Medical Research
- Fondation Bettencourt-Schueller
- Center for Research on Influenza Pathogenesis and Transmission
- 75N93021C00014/AI/NIAID NIH HHS/United States
