A Digital Patient Decision Aid to Increase Sexually Transmitted Infection Testing in the Emergency Department: Protocol for a Pilot Randomized Controlled Trial of the STIckER (STI Check in the Emergency Room) Study
- PMID: 41358448
- PMCID: PMC12683500
- DOI: 10.2196/67103
A Digital Patient Decision Aid to Increase Sexually Transmitted Infection Testing in the Emergency Department: Protocol for a Pilot Randomized Controlled Trial of the STIckER (STI Check in the Emergency Room) Study
Abstract
Background: Rising rates of sexually transmitted infections (STIs) among adolescents and young adults are concerning. Emergency department (ED) visits offer a unique opportunity to increase STI testing. However, providers in the ED may not have the time or self-efficacy to screen patients, and patients may be unable to initiate discussion on their STI care needs. A patient decision aid may improve the effectiveness and efficiency of the ED encounter for STI testing.
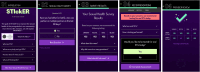
Objective: This study aims to pilot STI Check in the Emergency Room (STIckER) app, a shared decision-making aid for urinary, oral, and rectal gonorrhea and chlamydia testing among adolescents and young adults, and assess STIckER's efficacy and implementation outcomes in an adult and pediatric ED.
Methods: We are conducting a parallel-group, two-arm provider-randomized controlled pilot trial in the adult and pediatric EDs at an urban academic medical center. Fifty providers were randomized 1:1 to intervention (trained on STIckER) or control (standard of care). Adolescents and young adults aged 14-24 years, English-speaking, and sexually active within the past 6 months are recruited during eligible shifts and assigned to the study arm of their provider. The standard of care comparator consists of usual provider-initiated STI discussions and testing without access to the decision aid; control participants receive a QR code directing them to a simple landing page encouraging them to ask their provider about STI testing. The planned enrollment target was 44 providers and 140 adolescent and young adult participants. Primary outcome is preliminary efficacy, defined as the proportion of adolescents and young adults receiving gonorrhea and chlamydia testing based on electronic medical record (EMR) review. Secondary outcomes include extragenital testing uptake, STI positivity rates, decisional conflict, and perceived level of shared decision-making. Implementation outcomes include feasibility, acceptability, appropriateness (using acceptability of intervention measure, intervention appropriateness measure, and feasibility of intervention measure), and provider self-efficacy. Data will be analyzed using descriptive statistics and regression models, with mixed-effects models applied if clustering by provider is detected. Exploratory analyses will examine differences by patient age, gender, and sexual identity, and provider characteristics.
Results: Between September 2023 and July 2025, we completed recruitment of 44 providers and 139 adolescent and young adult participants across both adult and pediatric EDs. Enrollment targets were met, with high fidelity to intervention delivery and strong rates of survey completion by both patients and providers. Final data cleaning and analyses are underway, and primary results are expected to be available in late 2025.
Conclusions: This pilot trial will provide critical data on the feasibility, acceptability, and preliminary efficacy of a digital SDM tool to increase STI testing in the ED. Findings will inform the design of a future multisite trial and the potential for scaling STIckER to support equitable, patient-centered STI screening across diverse clinical settings.
Keywords: STI; adolescent sexual health; digital health; emergency department STI testing; mHealth; mobile apps; sexually transmitted infection; youth.
© Jason Zucker, Delivette Castor, Marc A Probst, Talia Adler, Lisa-Pierre Tchoungui, Jaciara De Souza, Christina Franqui, Lauren Chernick. Originally published in JMIR Research Protocols (https://www.researchprotocols.org).
Conflict of interest statement
Figures
References
-
- Sexually transmitted infections surveillance 2022. Centers for Disease Control and Prevention. 2024. [31-10-2025]. https://www.cdc.gov/sti-statistics/media/pdfs/2024/11/2022-STI-Surveilla... URL. Accessed.
-
- Cairns C, Kang K, Santo L. Centers for Disease Control and Prevention; [16-11-2025]. National Hospital Ambulatory Medical Care Survey: 2018 Emergency Department summary tables.https://www.cdc.gov/nchs/data/nhamcs/web_tables/2018-ed-web-tables-508.pdf URL. Accessed.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

