Discovery of a β-arrestin-biased CCKBR agonist that blocks CCKBR-dependent long-term potentiation
- PMID: 41360797
- PMCID: PMC12686490
- DOI: 10.1038/s41467-025-65962-y
Discovery of a β-arrestin-biased CCKBR agonist that blocks CCKBR-dependent long-term potentiation
Abstract
The CCKBR agonists induce neocortical long-term potentiation of excitatory synaptic transmission and enhance memory formation, while its antagonists weaken the potentiation in the amygdala and alleviate depression-like behaviors. However, the mechanism that drives CCKBR dependent long-term potentiation remains elusive. There is also no signaling pathway-biased CCKBR agonist to modulate the potentiation. Here, we discover a β-arrestin biased CCKBR agonist MF-8 with IC50 = 0.9 nM. The activation of CCKBR with MF-8 fails to induce the potentiation but efficiently induces CCKBR endocytosis. Multi-Electrode Array results demonstrate that the potentiation is dependent on Gαq/11-Ca2+ and Gαs-cAMP signaling pathways. The potentiation is entirely blocked by MF-8 through β-arrestin signaling. Furthermore, MF-8 effectively inhibits the formation of cue-to-cue associative fear memory. These results reveal the signal pathway preference of the CCKBR long-term potentiation and identify a blocker of the potentiation, which provides us with broader insights into developing drugs targeting CCKBR.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
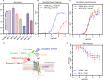
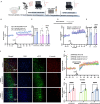

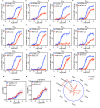






References
-
- Wang, W., Qiao, Y. & Li, Z. New insights into modes of GPCR activation. Trends Pharmacol. Sci.39, 367–386 (2018). - PubMed
-
- Calebiro, D., Koszegi, Z., Lanoiselée, Y., Miljus, T. & O’Brien, S. G protein-coupled receptor-G protein interactions: a single-molecule perspective. Physiol. Rev.101, 857–906 (2021). - PubMed
MeSH terms
Substances
Grants and funding
- CityUHK 11101521, CityUHK 11103922, CityUHK 11104923, CityUHK 11104524./Research Grants Council, University Grants Committee (RGC, UGC)
- C1043-21G/Research Grants Council, University Grants Committee (RGC, UGC)
- T13-605/18-W/Research Grants Council, University Grants Committee (RGC, UGC)
- SRFS2324-1S02/Research Grants Council, University Grants Committee (RGC, UGC)
LinkOut - more resources
Full Text Sources

