A bacteriophage genus infects carbapenem resistant Acinetobacter baumannii via a non-capsular receptor and provides protection in vivo
- PMID: 41362605
- PMCID: PMC12682139
- DOI: 10.1016/j.isci.2025.114026
A bacteriophage genus infects carbapenem resistant Acinetobacter baumannii via a non-capsular receptor and provides protection in vivo
Abstract
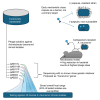
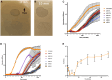
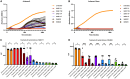
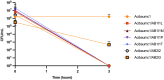
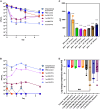
Extensive drug resistance (XDR) in Acinetobacter baumannii and other pathogens has revitalized bacteriophage as a therapeutic consideration. Six phages (AB1I1L, AB1I1M, AB1I1P, AB1I1T, AB2I2, and AB2I3) targeting A. baumannii were isolated from wastewater. These represent a previously undescribed phage genus with rapid adsorption and potent lysis. 18/40 A. baumannii clinical isolates, including 11/27 carbapenem-resistant isolates, were susceptible to one of the isolated phages. Importantly, in vitro-derived, phage resistant bacteria were killed in human ascites, demonstrating decreased biofitness. In contrast to most described phages that target A. baumannii, the bacterial capsule is not the primary receptor. Capsule impedes phage activity in vitro. The treatment of an XDR isolate using phage monotherapy in a rat subcutaneous abscess model showed dose-dependent efficacy, though a higher sustained concentration of phage was needed when compared with in vitro conditions. These phages are potential candidates for phage therapy, warranting additional preclinical evaluation as adjunctive treatment for A. baumannii infections.
Keywords: Microbiology; Virology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- CDC . CDC; Atlanta, GA: 2022. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022.
LinkOut - more resources
Full Text Sources

