Enhancing bovicin HC5 production in Streptococcus equinus HC5 through adaptive laboratory evolution under thermal stress
- PMID: 41366611
- PMCID: PMC12696137
- DOI: 10.1007/s00253-025-13642-8
Enhancing bovicin HC5 production in Streptococcus equinus HC5 through adaptive laboratory evolution under thermal stress
Abstract
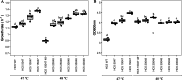
Bovicin HC5, a bacteriocin produced by Streptococcus equinus HC5, demonstrates inhibitory activity against pathogenic and spoilage microorganisms. However, low production yields hinder its widespread application. This study investigated the impact of temperature on S. equinus HC5 growth and employed adaptive laboratory evolution (ALE) under heat stress to obtain variants with improved bovicin HC5 production. The optimal growth temperature for the wild-type strain was determined to be 42 °C, with growth ceasing above 49 °C. Following 400 generations of ALE at 47 °C and 48 °C, eight variants were selected. Two of these variants exhibited significantly enhanced bovicin HC5 production, reaching up to a 140% increase (P < 0.05). The variant with the highest bacteriocin yield showed increased expression of bvcA, the gene encoding the bovicin HC5 precursor peptide. This high-producing variant also displayed enhanced thermal resistance, a higher growth rate (μ = 1.33 ± 0.02 h-1), and increased biomass accumulation (OD600nm = 4.03 ± 0.06) at 48 °C compared to the wild-type strain (μ = 0.98 ± 0.04 h-1; OD600nm = 1.96 ± 0.12) (P < 0.05). Furthermore, the selected variants exhibited alterations in membrane composition, characterized by an increased concentration of saturated fatty acids and a reduced Zeta potential (P < 0.05). Genomic analysis of these variants identified mutations in genes involved in protein modification, transcriptional regulation, and cellular transport, including a lantibiotic permease. These results demonstrate the effectiveness of ALE for generating S. equinus HC5 variants with improved bovicin HC5 production and provide valuable insights for optimizing bacteriocin biosynthesis strategies. KEY POINTS: • The optimal growth temperature for the Streptococcus equinus HC5 strain was determined to be 42 °C, with growth ceasing above 49 °C • The variant Streptococcus equinus HC5 40048 with the highest bacteriocin yield showed increased expression of bvcA, the gene encoding the bovicin HC5 precursor peptide • ALE is an efficient metabolic engineering strategy to increase bacteriocin production in Streptococcus equinus HC5.
Keywords: Antimicrobial peptides; Comparative genomics; Natural selection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Competing interest: The authors declare no competing interests.
Figures







References
-
- Alkhatib Z, Abts A, Mavaro A, Schmitt L, Smits SHJ (2012) Lantibiotics: how do producers become self-protected? J Biotechnol 159(3):145–154. 10.1016/j.jbiotec.2012.01.032 - PubMed
-
- Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute. https://www.bioinformatics.babraham.ac.uk/projects/fastqc
-
- Asanuma N, Hino T (2002) Regulation of fermentation in a ruminal bacterium, Streptococcus bovis, with special reference to rumen acidosis. Anim Sci J 73(4):313–325. 10.1046/j.1344-3941.2002.00044.x
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

