Mechanisms and Impact of Cognitive Reserve in Normal Aging and Alzheimer's Disease
- PMID: 41374449
- PMCID: PMC12692465
- DOI: 10.3390/diagnostics15233068
Mechanisms and Impact of Cognitive Reserve in Normal Aging and Alzheimer's Disease
Abstract
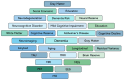
Age-related cognitive decline and individual differences in dementia susceptibility are increasingly explained through the concept of cognitive reserve (CR). CR reflected the brain's adaptive capacity to sustain cognitive performance despite Alzheimer's disease (AD)-related pathology, extending beyond traditional biomarkers that captured the molecular or structural changes, but often failed to account for clinical heterogeneity. This review provided a comprehensive synthesis of how CR was operationalized through three major methodological approaches: sociobehavioral proxies, residual variance frameworks, and neurobiological indicators within the context of longitudinal study designs. The review included evidences from a structured PubMed and Scopus search restricted to English-language studies examining the incidence of mild cognitive impairment (MCI) or AD. Findings consistently demonstrated that higher CR, most commonly estimated through sociobehavioral proxies, such as educational level, occupational complexity, bilingualism, and engagement in cognitively stimulating activities, was associated with a delayed onset of impairment, lower dementia risk, and better clinical outcomes, despite a comparable neuropathological burden. Residual variance approaches provided complementary insights by quantifying cognitive performance that exceeded the predicted levels from underlying pathology, thereby capturing unexplained variance by structural or molecular disease markers. These residual-based methods extend CR concept beyond life-course experiences, offering statistical evidence of resilience within longitudinal trajectories of aging and disease. Additional evidence from electrophysiological and genetic investigations further suggested that CR enhanced the neural efficiency, flexibility, and the recruitment of compensatory networks. Finally, neuroimaging studies provided the mechanistic evidence that CR was supported by alterations in brain structure, functional connectivity, and activation patterns, though findings on long-term trajectories remained inconsistent. Overall, CR emerged as a multidimensional and modifiable construct that enhanced resilience to aging and dementia. Future research should prioritize the integrative longitudinal designs, combining sociobehavioral, residual variance, genetic, electrophysiological, and neuroimaging approaches to clarify mechanisms, establishing robust measurement frameworks and advance clinical translation.
Keywords: Alzheimer’s disease; aging; biomarkers; cognitive reserve; mild cognitive impairment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G., Dubois B., Dufouil C., Ellis K.A., van der Flier W.M., et al. Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. - DOI - PMC - PubMed
-
- Molinuevo J.L., Rabin L.A., Amariglio R., Buckley R., Dubois B., Ellis K.A., Ewers M., Hampel H., Klöppel S., Rami L., et al. Subjective Cognitive Decline Initiative (SCD-I) Working Group. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017;13:296–311. doi: 10.1016/j.jalz.2016.09.012. - DOI - PMC - PubMed
Publication types
Grants and funding
- 2021R1A6A1A03038996/Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education
- RS-2024-00507796/Technology Innovation Program grants through Korea Planning & Evaluation Institute of Industrial Technology (KEIT) funded by the Ministry of Trade, Industry & Energy
LinkOut - more resources
Full Text Sources

