Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale
- PMID: 4200894
- PMCID: PMC6945976
- DOI: 10.1021/bi00745a021
Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale
Abstract
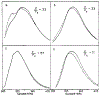
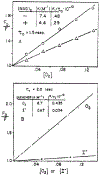
Quenching of the tryptophan fluorescence of native proteins was studied using oxygen concentrations up to 0.13
Figures



References
-
- Arnon R, and Perlmann GE (1963), J. Biol. Chem 238, 653.
-
- Armstrong McD. J., Myers DV, Verpoorte JA, and Edsall JT (1966),J. Biol. Chem 241, 5137. - PubMed
-
- Bakhshiev NG (1964), Opt. Spectrosc. (USSR) 16,446.
-
- Brand L, and Gohlke JR (1971), J. Biol. Chem 246,2317. - PubMed
-
- Brandts JF, Oliveira RJ, and Westort C (1970), Biochemistry 9,1038. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
