Fluorescence anisotropy measurements under oxygen quenching conditions as a method to quantify the depolarizing rotations of fluorophores. Application to diphenylhexatriene in isotropic solvents and in lipid bilayers
- PMID: 420798
- PMCID: PMC6827200
- DOI: 10.1021/bi00570a022
Fluorescence anisotropy measurements under oxygen quenching conditions as a method to quantify the depolarizing rotations of fluorophores. Application to diphenylhexatriene in isotropic solvents and in lipid bilayers
Abstract
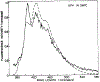
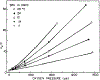
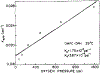
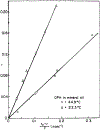
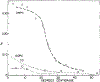
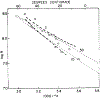
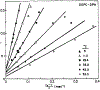
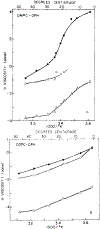
We have measured the fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH) as its fluorescence lifetime is decreased by oxygen quenching. Such studies were done on DPH dissolved in the isotropic solvent mineral oil and for DPH embedded in phospholipid vesicles of either dimyristoyl-
Figures

References
-
- Anderson SR, & Weber G (1965) Biochemistry 4, 1948.
-
- Boaz H, & Rollefson GK (1950) J. Am. Chem. Soc 72, 3435.
-
- Bridge JB, & Johnson P (1973) Eur. Polym. J 9, 1327.
-
- Chen LA, Dale RE, Roth S, & Brand L (1977) J. Biol. Chem 252, 2163. - PubMed
-
- Cogan V, Shinitzky M, Weber G, & Nishida T (1973) Biochemistry 12, 521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
