The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood
- PMID: 4353133
- PMCID: PMC2747591
The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood
Figures

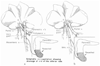






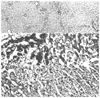







References
-
- Albano O, Francavilla A. Effect of CC14 intoxication in the synthesis of β-Iipoprotein fractions in rat liver. Clin. Chim. Acta. 1968;19:363. - PubMed
-
- Barron EJ, Hanahan DJ. Observations on the silicic acid chromatography of the neutral Iipides of rat liver, beef liver, and yeast. J. Biol. Chem. 1958;231:493. - PubMed
-
- Bergen SS, Hilton JG, Van Itallie TB. Glycogenolytic effect of adenosine 3′–5′-monophosphate in the canine liver. Endocrinology. 1966;79:1065. - PubMed
-
- Bloom WL, Lewis GT, Schumpert MZ, Shen T-M. Glycogen fractions of liver and muscle. J. Biol. Chem. 1951;188:631. - PubMed
-
- Bocher NLR. Experimental aspects of hepatic regeneration. N. Engl. J. Med. 1967;277:686. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
