Microvasculature of the dog left ventricular myocardium
- PMID: 4596001
- PMCID: PMC3175795
- DOI: 10.1016/0026-2862(74)90008-9
Microvasculature of the dog left ventricular myocardium
Abstract
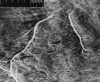
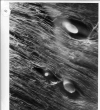
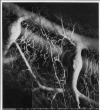
One of the main branches of the left main coronary artery of normally beating dog hearts was perfused with a silicone elastomer which solidified within the vasculature. Prolonged immersion in increasingly concentrated ethanol and in methyl salicylate rendered the tissue translucent and the vasculature clearly visible. Surfaces were photographed by reflected or transmitted light microscopy, showing large groups of capillaries running parallel to muscle fibers and extending for up to a few centimeters. The arrangement of arteriolar inflows to the capillary network and venular outflows (two to four times as frequent) suggested that functional capillary lengths were 500–1000 μm. Estimates of capillary diameters, presumably at maximal dilatation, were 5.6 ± 1.3 μm. Capillary densities within muscle groups were 3100–3800/mm2, giving intercapillary distances of 19–17.5 μm. With the lesser density value, the capillary surface area is estimated to be 500 cm2/g of myocardium. Inclusion of interfascial spaces lowered the average density to about 2500/mm2. Unbranched capillary lengths averaged 100 μm, with a strongly right-skewed distribution. The anatomic arrangement provides a basis mainly for concurrent flow in neighboring capillaries, and also for some diffusional exchange between inflow and outflow regions.
Figures






References
-
- Bassingthwaighte JB, Strandell T, Yipintsoi T. Flow limited washout of diffusible solutes from the heart. In: Crone C, Lassen NA, editors. Capillary Permeability. Munksgaard; Copenhagen: 1970. pp. 580–585.
-
- Bassingthwaighte JB, Yipintsoi T. Countercurrent exchange of water in the heart. Biophys J. 1969;9:232A.
-
- Detar R, Bohr DF. Oxygen and vascular smooth muscle contraction. Amer J Physiol. 1968;214:241–244. - PubMed
-
- Fabel H. Normal and critical O2 supply of the heart. In: Lubbers DW, Luft UC, Thews G, Witzleb E, editors. Oxygen Transport in Blood and Tissue. Georg Thieme Verlag; Stuttgart, Germany: 1968. pp. 159–171.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
