Progress in homotransplantation of the liver
- PMID: 5333482
- PMCID: PMC2975435
Progress in homotransplantation of the liver
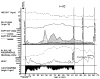
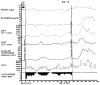
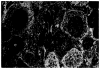
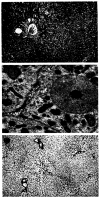
Figures







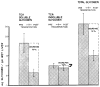
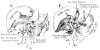


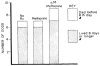

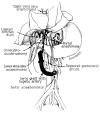



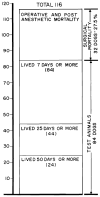
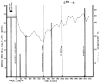
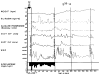

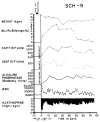
















References
-
- Absolon KB. Personal communication. 1964.
-
- Anderson MC, Bergan JJ, Salan JR. Experimental production of hepatocellular damage with cortisone. S Forum. 1960;11:13. - PubMed
-
- Arey LB. Throttling veins in the livers of certain mammals. Anat Rec. 1941;81:21.
-
- Baker BL, et al. The effect on liver structure of treatment with adreno-corticotropin under varied dietary conditions. Am J Anat. 1948;82:79. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
