The Eck fistula in animals and humans
- PMID: 6357642
- PMCID: PMC3154772
- DOI: 10.1016/s0011-3840(83)80010-0
The Eck fistula in animals and humans
Abstract
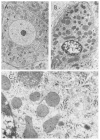
In all species so far studied, including man, portacaval shunt causes the same changes in liver morphology, including hepatocyte atrophy, fatty infiltration, deglycogenation, depletion and disorganization of the rough endoplasmic reticulum (RER) and its lining polyribosomes, and variable but less specific damage to other organelles. Many, perhaps all, biosynthetic processes are quickly depressed, largely secondary to the selective damage to the RER, which is the "factory" of the cell. These structural and metabolic changes in the liver after portal diversion are caused by the diversion around the liver of the hepatotrophic substances in portal venous blood, of which endogenous insulin is the most important. In experimental animals, the injury of Eck's fistula can be prevented by infusing insulin into the tied-off hilar portal vein. The subtle but far-reaching changes in hepatic function after portal diversion have made it possible to use this procedure in palliating three inborn errors of metabolism: glycogen storage disease, familial hypercholesterolemia, and alpha 1-antitrypsin deficiency. In these three diseases, the abnormalities caused by portal diversion have counteracted abnormalities in the patients that were caused by the inborn errors. In these diseases, amelioration of the inborn errors depends on the completeness of the portal diversion. In contrast, total portal diversion to treat complications of portal hypertension is undesirable and always will degrade hepatic function if a significant amount of hepatopetal portal venous blood is taken from the liver. When total portal diversion is achieved (and this is to be expected after all conventional shunts), the incidence of hepatic failure and hepatic encephalopathy is increased. If portal diversion must be done for the control of variceal hemorrhage, a selective procedure such as the Warren procedure is theoretically superior to the completely diverting shunt. In practice, better patient survival has not been achieved after selective shunts than after conventional shunts, but the incidence of hepatic encephalopathy has been less.
Figures













Similar articles
-
The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons.Surg Gynecol Obstet. 1975 Mar;140(3):381- 96. Surg Gynecol Obstet. 1975. PMID: 18689027 Free PMC article.
-
Screening for candidate hepatic growth factors by selective portal infusion after canine Eck's fistula.Hepatology. 1991 Oct;14(4 Pt 1):665-70. doi: 10.1016/0270-9139(91)90055-z. Hepatology. 1991. PMID: 1916668 Free PMC article.
-
Reversal of hepatic alpha-1-antitrypsin deposition after portacaval shunt.Lancet. 1983 Aug 20;2(8347):424-6. doi: 10.1016/s0140-6736(83)90390-2. Lancet. 1983. PMID: 6135912 Free PMC article.
-
Portal hypertension: from Eck's fistula to TIPS.Ann Surg. 2003 Dec;238(6 Suppl):S49-55. doi: 10.1097/01.sla.0000097526.74747.90. Ann Surg. 2003. PMID: 14703745 Review.
-
Developments in management of portal hypertension: a plea for selective symptomatic treatment.Surg Annu. 1980;12:317-39. Surg Annu. 1980. PMID: 6996172 Review. No abstract available.
Cited by
-
Survival of human hepatocellular aggregates in athymic mice.Transplant Proc. 1992 Jun;24(3):986. Transplant Proc. 1992. PMID: 1604699 Free PMC article. No abstract available.
-
Current status of intestinal transplantation.Adv Surg. 1994;27:295-316. Adv Surg. 1994. PMID: 8140977 Free PMC article. Review. No abstract available.
-
Maximizing efficacy from parenteral nutrition in critical care: appropriate patient populations, supplemental parenteral nutrition, glucose control, parenteral glutamine, and alternative fat sources.Curr Gastroenterol Rep. 2007 Aug;9(4):345-53. doi: 10.1007/s11894-007-0040-1. Curr Gastroenterol Rep. 2007. PMID: 17883985 Review.
-
Preclinical models of acute liver failure: a comprehensive review.PeerJ. 2021 Dec 9;9:e12579. doi: 10.7717/peerj.12579. eCollection 2021. PeerJ. 2021. PMID: 34966588 Free PMC article.
-
Small bowel transplantation. A life-saving option for selected patients with intestinal failure.Dig Dis Sci. 1996 May;41(5):875-83. doi: 10.1007/BF02091526. Dig Dis Sci. 1996. PMID: 8625758
References
-
- Starzl TE, Porter KA, Francavilla A, et al. 100 years of the hepatotrophic controversy. In: Porter R, Whelan J, editors. Hepatotrophic Factors; Ciba Foundation Symposium No. 55; Amsterdam: Excerpta Medica (Elsevier North-Holland); 1978. pp. 111–129. - PubMed
-
- Starzl TE, Terblanche J. Hepatotrophic substances. Prog Liver Dis. 1979;6:135–152. - PubMed
-
- Eck NV. Voen Med J. Vol. 130. 1877. K voprosu o perevyazkie vorotnois veni: Predvaritelnoye soobschjenye; pp. 1–2. (Ligature of the portal vein, trans. Child C.G. III: Surg. Gynecol. Obstet. 96:375–376, 1953.)
-
- Hahn M, Massen O, Nencki M, Pawlow J. Die Eck’sche Fistel zwischen der unteren Hohlvene und der Pfortader und ihre Folgen für den Organismus. Arch Exp Pathol Pharmakol. 1893;32:161–210.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

