Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin
- PMID: 6450206
- PMCID: PMC1351010
Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin
Abstract
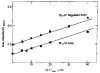
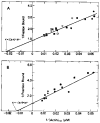
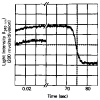
The regulation of vertebrate skeletal muscle contraction by the troponin . tropomyosin complex is generally thought to be the result of tropomyosin physically blocking the myosin binding site of actin in the absence of Ca2+. This mechanism was tested during steady state ATP hydrolysis by comparing the degree of association of myosin subfragment 1 (S-1) with the actin . troponin . tropomyosin complex in the absence and presence of Ca2+. Binding in the presence of ATP was determined by stopped flow absorbance measurements at 25 degrees C. Although the steady state ATPase rate was reduced 96% in the absence of Ca2+, the association constant of S-1 with regulated actin was virtually the same in the absence of Ca2+ (1.3 X 10(4) M-1) as in the presence of Ca2+ (2.3 X 10(4) M-1). The association constant of S-1 to regulated actin in the presence of Ca2+ was similar to the association constant of S-1 to unregulated actin. These results suggest that the troponin . tropomyosin complex does not inhibit the actin-activated ATPase activity by preventing the binding of S-1 . ATP or S-1 . ADP . Pi to actin; rather, it may act by blocking the release of Pi from the acto-S-1 . ADP . Pi complex.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

