Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin
- PMID: 6460759
- PMCID: PMC1266292
Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin
Abstract
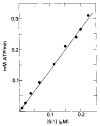
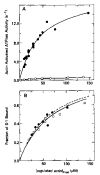
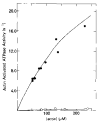
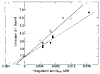
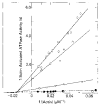
Relaxation of vertebrate skeletal muscle is thought to occur in the absence of Ca2+ as a result of tropomyosin physically blocking the binding of myosin to actin. This steric blocking model of muscle relaxation predicts that myosin subfragment 1 (S-1) will not bind to actin under conditions where the acto-S-1 ATPase rate is inhibited. Using stopped flow absorbance as a measure of binding, we have previously shown that when the rate of ATP hydrolysis is only 4% of the rate in the presence of Ca2+, S-1·ATP and S-1·ADP·Pi bind to actin-troponin-tropomyosin (regulated actin) with almost the same affinity as in the presence of Ca2+. This result has now been confirmed using sedimentation in an air-driven ultracentrifuge to directly measure the binding at pH 7.0, 25 °C, and μ = 18 m
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

