Diffusional arteriovenous shunting in the heart
- PMID: 6503735
- PMCID: PMC3175769
- DOI: 10.1016/0026-2862(84)90020-7
Diffusional arteriovenous shunting in the heart
Abstract
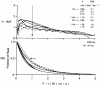
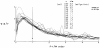
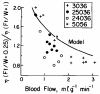
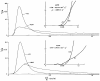
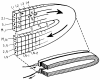
Previous indicator dilution experiments in isolated blood-perfused dog hearts suggested that there was intramyocardial diffusional shunting of water relative to a flow-limited solute, antipyrine. Two sets of studies have been done to assess the importance of this shunting, since it implies the possibility of a diffusional bypass for oxygen and other substances, which may be important in ischemia. Nonconsumed tracers were used to show the phenomenon. In the first set, bolus injections of 133Xe dissolved in saline were made into the coronary inflow and the tracer content of the organ recorded by an external gamma detector. The initial Xe washout was disproportionately rapid at low flows, and the late phase was also relatively retarded. In the second set, boluses of cool saline containing indocyanine green were injected into the coronary arterial inflow while coronary sinus outflow dilution curves were recorded via a thermistor and a dye densitometer over a wide range of flows. The thermal curves showed emergence of heat preceding the dye; the degree of precession was much greater at low flows, and, unlike the dye curves, the thermal dilution curves showed dramatic differences in shape at different flows. A model for diffusional countercurrent exchange shows similar changes in residue curves and outflow dilution curves. The conclusion is that there is diffusional shunting of small lipid-soluble molecules whose diffusion coefficients in tissue are high. While the shunting of heat is great, the shunting of soluble gases will not be large and that of normal substrates will be negligible.
Figures
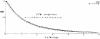


References
-
- Armour JA, Klassen GA. Epicardial coronary venous pressure. Canad. J. Physiol. Pharmacol. 1981;59:1250–1259. - PubMed
-
- Aukland K. Renal medullary heat clearance in the dog. Circ. Res. 1967;20:194–203. - PubMed
-
- Aukland K, Berliner RW. Renal medullary countercurrent system studied with hydrogen gas. Circ. Res. 1964;15:430–442. - PubMed
-
- Aukland K, Bower BF, Berliner RW. Measurement of local blood flow with hydrogen gas. Circ. Res. 1964;14:164–187. - PubMed
-
- Barlow CH, Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science. 1976;3:909–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

