Regional specialization of retinal glial cell membrane
- PMID: 6717594
- PMCID: PMC2693194
- DOI: 10.1038/309155a0
Regional specialization of retinal glial cell membrane
Abstract
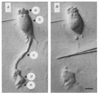
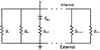
Neural activity generates increases in extracellular K+ concentration, [K+]0, which must be regulated in order to maintain normal brain function. Glial cells are thought to play an important part in this regulation through the process of K+ spatial buffering: K+-mediated current flow through glial cells redistributes extracellular K+ following localized [K+]0 increases. As is the case in other glia, the retinal Müller cell is permeable almost exclusively to K+ . Recent experiments have suggested that this K+ conductance may not be distributed uniformly over the cell surface. In the present study, two novel techniques have been used to assess the Müller cell K+ conductance distribution. The results demonstrate that 94% of all membrane conductance lies in the endfoot process of the cell. This strikingly asymmetric distribution has important consequences for theories concerning K+ buffering and should help to explain the generation of the electroretinogram.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

