Synthesis and use of radio cobaltic EDTA as an extracellular marker in rabbit heart
- PMID: 6802001
- PMCID: PMC3010220
- DOI: 10.1152/ajpheart.1982.242.4.H671
Synthesis and use of radio cobaltic EDTA as an extracellular marker in rabbit heart
Abstract
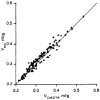
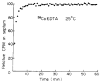
A new gamma-labeled marker for extracellular space is the cobaltic form of 58Co-ethylenediaminetetraacetic acid (58Co-EDTA). The cobaltic ion has a much higher affinity for EDTA than the cobaltous ion; it is prepared as a potassium salt, K+(58Co3+-EDTA4-), and is apparently biologically inert. Testing by equilibration in intact rabbits and comparing the myocardial content with that of [14C]sucrose give values of the volume of distribution in the myocardium of 0.294 +/- 0.052 ml/g for 58Co-EDTA and 0.303 +/- 0.051 ml/g for [14C]sucrose (SD, n = 130, for two hearts), with the ratios of 58Co-EDTA/sucrose averaging 0.973 +/- 0.043 (n = 130). The average value of the extracellular fluid measured in isolated rabbit interventricular septum using Co-EDTA was 0.51 +/- 0.05 ml/g (SD, n = 16) and 0.46 +/- 0.04 ml/g using [14C]sucrose as an extracellular fluid space (ECF) marker. Flushing with a high concentration of nontracer Co-EDTA does not reveal any release from binding sites. The gamma-energy (811 KeV), long half-life (71.4 days), stability, and lack of binding to tissue components make 58Co-EDTA a useful marker for ECF.
Figures




References
-
- Bjerrum J, Schwartzenbach G, Sillen LG. Stability constants of metal ion complexes. London: Chem Soc. 1957:76–77.
-
- Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. - PubMed
-
- Dwyer FP, Gyarfas E, Mellor D. The resolution of racemization of potassium ethylenediance tetracetate cobaltate III. J Phys Chem. 1955;59:296–297.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

