Pilot trial of FK 506 in the management of steroid-resistant nephrotic syndrome
- PMID: 7508098
- PMCID: PMC2974461
Pilot trial of FK 506 in the management of steroid-resistant nephrotic syndrome
Abstract
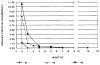
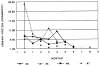
Seven patients with steroid-resistant nephrotic syndrome were treated with FK 506 monotherapy. Four patients were children with focal sclerosing glomerulonephritis (FSGS). Three of these had evidence for chronic progressive renal disease consisting of interstitial fibrosis and tubular atrophy on pretreatment renal biopsies. Two patients had also failed cyclosporin A (CsA), two cyclophosphamide, and one chlorambucil prior to treatment with FK 506. Three patients were adults with mesangial proliferative, membranoproliferative, and membranous glomerulonephritis. Three patterns of response were noted: (1) a reduction in proteinuria to normal levels; (2) partial response (50% reduction) or; (3) no improvement. All patients except one experienced at least a 50% reduction in protein excretion at some time during FK 506 therapy. Two of the children and one adult reduced protein excretion to essentially normal values. One patient had no sustained reduction in protein excretion and is considered to be a treatment failure, although her protein excretion was approximately 50% of pretreatment values intermittently. The drug was generally well tolerated. The most common side-effect was nephrotoxicity, which was reversible. These encouraging results suggest that FK 506 monotherapy may be effective in controlling the proteinuria of some patients with steroid-resistant nephrotic syndrome. The use of this drug may extend our understanding of the role of T lymphocytes and cytokines in the pathogenesis of glomerulonephritis. Further study of this agent in a larger population of patients is warranted.
Figures


References
-
- Brodehl J. Conventional therapy for idiopathic nephrotic syndrome in children. Clin Nephrol. 1991;35:s8–15. - PubMed
-
- Ponticelli C, Passerini P. Conventional treatment of idiopathic nephrotic syndrome and membranous nephropathy in adults. Clin Nephrol. 1991;35:s16–s21. - PubMed
-
- Meyrier A, Simon P. Advances in Nephrology. Yearbook Medical Publishers; Chicago: 1988. Treatment of corticoresistant idopathic nephrotic syndrome in adult: minimal change disease and focal segmental glomerulosclerosis; pp. 127–150. - PubMed
-
- Korbet SM, Schwartz MM, Lewis EJ. The prognosis of focal segmental glomerular sclerosis of adulthood. Medicine. 1986;65:304–311. - PubMed
-
- Pei Y, Cattran D, Delmore T, Katz A, Lang A, Range P. Evidence suggesting under-treatment in adults with idiopathic focal segmental glomerulosclerosis. Regional glomerulonephritis registry study. Am J Med. 1987;82:938–944. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
