Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis
- PMID: 7510257
- PMCID: PMC6520052
- DOI: 10.1101/gad.8.4.440
Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis
Abstract
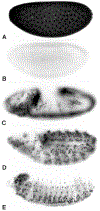
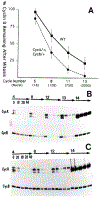
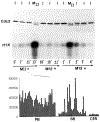
The conserved regulators of cell cycle progression--Cyclins, Cdc2 kinase, and String phosphatase (Cdc25)--accommodate multiple modes of regulation during Drosophila embryogenesis. During cell cycles 2-7, Cdc2/Cyclin complexes are continuously present and show little fluctuation in abundance, phosphomodification, or activity. This suggests that cycling of the mitotic apparatus does not require cytoplasmic oscillations of known regulatory activities. During cycles 8-13 a progressive increase in the degradation of Cyclins at mitosis leads to increasing oscillations of Cdc2 kinase activity. Mutants deficient in cyclin mRNAs suffer cell cycle delays during this period, suggesting that Cyclin accumulation times these cycles. During interphase 14, programmed degradation of maternal String protein leads to inhibitory phosphorylation of Cdc2 and cell cycle arrest. Subsequently, mitoses 14-16 are triggered by pulses of zygotic string transcription.
Figures


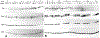

References
-
- Alfa CE, Ducommun B, Beach D, and Hyams JS. 1990. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature 347: 680–682. - PubMed
-
- Amon A, Surana U, Muroff I, and Nasmyth K. 1992. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature 355: 368–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
