Specific roles of the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins in avian neural crest cell adhesion and migration on vitronectin
- PMID: 7525179
- PMCID: PMC2710119
- DOI: 10.1242/dev.120.9.2687
Specific roles of the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins in avian neural crest cell adhesion and migration on vitronectin
Abstract
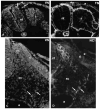
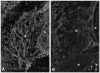
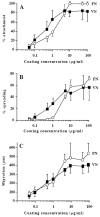
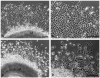
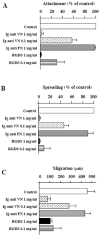
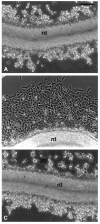
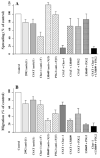
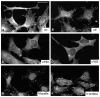
To identify potentially important extracellular matrix adhesive molecules in neural crest cell migration, the possible role of vitronectin and its corresponding integrin receptors was examined in the adhesion and migration of avian neural crest cells in vitro. Adhesion and migration on vitronectin were comparable to those found on fibronectin and could be almost entirely abolished by antibodies against vitronectin and by RGD peptides. Immunoprecipitation and immunocytochemistry analyses revealed that neural crest cells expressed primarily the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins as possible vitronectin receptors. Inhibition assays of cellular adhesion and migration with function-perturbing antibodies demonstrated that adhesion of neural crest cells to vitronectin was mediated essentially by one or more of the different alpha V integrins, with a possible preeminence of alpha V beta 1, whereas cell migration involved mostly the alpha V beta 3 and alpha V beta 5 integrins. Immunofluorescence labeling of cultured motile neural crest cells revealed that the alpha V integrins are differentially distributed on the cell surface. The beta 1 and alpha V subunits were both diffuse on the surface of cells and in focal adhesion sites in association with vinculin, talin and alpha-actinin, whereas the alpha V beta 3 and alpha V beta 5 integrins were essentially diffuse on the cell surface. Finally, vitronectin could be detected by immunoblotting and immunohistochemistry in the early embryo during the ontogeny of the neural crest. It was in particular closely associated with the surface of migrating neural crest cells. In conclusion, our study indicates that neural crest cells can adhere to and migrate on vitronectin in vitro by an RGD-dependent mechanism involving at least the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins and that these integrins may have specific roles in the control of cell adhesion and migration.
Figures



References
-
- Barnes DW, Reing JE, Amos B. Heparin-binding properties of human serum spreading factor. J Biol Chem. 1985;260:9117–9122. - PubMed
-
- Bodary SC, McLean JW. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. - PubMed
-
- Boucaut J-C, Darribère T, Poole TJ, Aoyama H, Yamada KM, Thiery JP. Biological active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984;99:1822–1830. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

