Tacrolimus (FK 506), a treatment for primary sclerosing cholangitis: results of an open-label preliminary trial
- PMID: 7532912
- PMCID: PMC2982698
Tacrolimus (FK 506), a treatment for primary sclerosing cholangitis: results of an open-label preliminary trial
Abstract
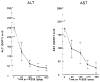
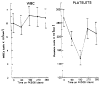
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease of the liver that is characterized by progressive cholestasis and the development of secondary biliary cirrhosis. There is no widely recognized therapy for this disease, although anti-inflammatory agents (steroids), immunosuppressive agents (methotrexate), anti-fibrotics (colchicine), and choleretic agents (ursodeoxycholic acid) have been used in various small series. In the present study, Tacrolimus (FK 506), a new and powerful immunosuppressive macrolide antibiotic, has been used to treat 10 patients with PSC. Each subject had a liver biopsy, ERCP with visualization of the intra- and extrahepatic biliary tree, and a panel of hematological, serological, and biochemical laboratory tests before the initiation of the FK 506 therapy. The FK 506 was administered orally at 12-h intervals and was monitored by serial plasma FK 506 trough levels. After 360 days of treatment, the median serum bilirubin level was reduced by 75%, and the serum alkaline phosphatase was reduced by 70%. Moreover, the serum ALT and AST levels were reduced by 80 and 86%, respectively. No change in the serum level of BUN and creatinine levels occurred as a consequence of the FK 506 treatment. These data demonstrate that: 1) FK 506 can be used to treat PSC; 2) the response to FK 506 by patients with PSC is rapid; and, 3) no adverse effect on the serum BUN and creatinine levels was observed. It is anticipated that FK 506 will become an important agent for the treatment of patients with PSC because of its powerful immunosuppressive activity.
Figures


Similar articles
-
Predictors of successful clinical and laboratory outcomes in patients with primary sclerosing cholangitis undergoing endoscopic retrograde cholangiopancreatography.Can J Gastroenterol. 2003 Apr;17(4):243-8. doi: 10.1155/2003/475603. Can J Gastroenterol. 2003. PMID: 12704468
-
Efficacy and safety of curcumin in primary sclerosing cholangitis: an open label pilot study.Scand J Gastroenterol. 2019 May;54(5):633-639. doi: 10.1080/00365521.2019.1611917. Epub 2019 May 26. Scand J Gastroenterol. 2019. PMID: 31131678 Free PMC article. Clinical Trial.
-
Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial.Am J Gastroenterol. 1995 May;90(5):771-6. Am J Gastroenterol. 1995. PMID: 7537444 Free PMC article. Clinical Trial.
-
[Novel Insights of Primary Sclerosing Cholangitis and Primary Biliary Cholangitis].Korean J Gastroenterol. 2020 May 25;75(5):246-256. doi: 10.4166/kjg.2020.75.5.246. Korean J Gastroenterol. 2020. PMID: 32448856 Review. Korean.
-
[Treatment of cholestatic liver disease].Rev Med Chir Soc Med Nat Iasi. 2003 Oct-Dec;107(4):733-6. Rev Med Chir Soc Med Nat Iasi. 2003. PMID: 14756010 Review. Romanian.
Cited by
-
Primary sclerosing cholangitis.Clin Rev Allergy Immunol. 2000 Apr;18(2):185-214. doi: 10.1385/CRIAI:18:2:185. Clin Rev Allergy Immunol. 2000. PMID: 10944705 Review. No abstract available.
-
Primary sclerosing cholangitis.Can J Gastroenterol. 2008 Aug;22(8):689-98. doi: 10.1155/2008/824168. Can J Gastroenterol. 2008. PMID: 18701947 Free PMC article. Review.
-
Clinical features and management of primary sclerosing cholangitis.World J Gastroenterol. 2008 Jun 7;14(21):3338-49. doi: 10.3748/wjg.14.3338. World J Gastroenterol. 2008. PMID: 18528931 Free PMC article. Review.
-
The medical management of primary sclerosing cholangitis.Medscape J Med. 2008 Mar 12;10(3):61. Medscape J Med. 2008. PMID: 18449341 Free PMC article. Review.
-
Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities.J Gastroenterol. 2020 Jun;55(6):588-614. doi: 10.1007/s00535-020-01681-z. Epub 2020 Mar 28. J Gastroenterol. 2020. PMID: 32222826 Free PMC article. Review.
References
-
- Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–6. - PubMed
-
- Schrupf E, Fansa O, Kolnanmskog F. Sclerosing cholangitis in ulcerative colitis: A follow-up study. Scand J Gastroenterol. 1982;17:33–9. - PubMed
-
- LaRusso NF, Wiesner RH, Ludwig J. Primary sclerosing cholangitis. N Engl J Med. 1984;310:899–903. - PubMed
-
- Sherlock S. The syndrome of disappearing bile ducts. Lancet. 1987;2:493–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
