Identification of a stable RNA encoded by the H-strand of the mouse mitochondrial D-loop region and a conserved sequence motif immediately upstream of its polyadenylation site
- PMID: 7537562
- PMCID: PMC6134379
Identification of a stable RNA encoded by the H-strand of the mouse mitochondrial D-loop region and a conserved sequence motif immediately upstream of its polyadenylation site
Abstract
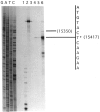
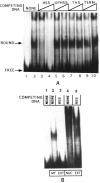
By using a combination of Northern blot hybridization with strand-specific DNA probes, S1 nuclease protection, and sequencing of oligo-dT-primed cDNA clones, we have identified a 0.8 kb poly(A)-containing RNA encoded by the H-strand of the mouse mitochondrial D-loop region. The 5' end of the RNA maps to nucleotide 15417, a region complementary to the start of tRNA(Pro) gene and the 3' polyadenylated end maps to nucleotide 16295 of the genome, immediately upstream of tRNA(Phe) gene. The H-strand D-loop region encoded transcripts of similar size are also detected in other vertebrate systems. In the mouse, rat, and human systems, the 3' ends of the D-loop encoded RNA are preceded by conserved sequences AAUAAA, AAUUAA, or AACUAA, that resemble the polyadenylation signal. The steady-state level of the RNA is generally low in dividing or in vitro cultured cells, and markedly higher in differentiated tissues like liver, kidney, heart, and brain. Furthermore, an over 10-fold increase in the level of this RNA is observed during the induced differentiation of C2C12 mouse myoblast cells into myotubes. These results suggest that the D-loop H-strand encoded RNA may have yet unknown biological functions. A 20 base pair DNA sequence from the 3' terminal region containing the conserved sequence motif binds to a protein from the mitochondrial extracts in a sequence-specific manner. The binding specificity of this protein is distinctly different from the previously characterized H-strand DNA termination sequence in the D-loop or the H-strand transcription terminator immediately downstream of the 16S rRNA gene. Thus, we have characterized a novel poly(A)-containing RNA encoded by the H-strand of the mitochondrial D-loop region and also identified the putative ultimate termination site for the H-strand transcription.
Figures









References
-
- Aloni Y. and Attardi G. (1971), J Mol Biol 28, 251–267. - PubMed
-
- Anderson S., Bankier A. T., Barrell B. G., de-Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F., Schreier P. H., Smith A. J., Staden R., and Young I. G. (1981), Nature 290, 457–465. - PubMed
-
- Anderson S., de-Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., and Young I. G. (1982), J Mol Biol 156, 683–717. - PubMed
-
- Attardi G. and Schatz G. (1988), Annu Rev Cell Biol 4, 289–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
