Translation-targeted therapeutics for viral diseases
- PMID: 7549467
- PMCID: PMC6134364
Translation-targeted therapeutics for viral diseases
Abstract
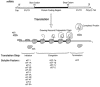
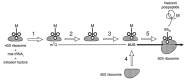
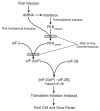
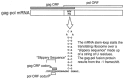
Viruses utilize the protein synthetic machinery of their host. Nonetheless, certain features of the synthesis of viral proteins are distinct from those of host-cell translation. Examples include internal ribosome entry sites in some viral mRNAs and ribosomal frameshifting during production of retroviral proteins. Viruses often inhibit host translation and/or possess mechanisms leading to preferential synthesis of viral proteins. In addition, a participant in the cellular antiviral response is the enzyme PKR (protein kinase, RNA activated), which is involved in the control of cellular translation. Thus, viruses and host cells wage war on the battlefield of translation. The distinctive features of protein synthesis in virally infected cells provide potential targets for therapeutic intervention. Translation-targeted therapeutics have precedence in antibiotics like tetracycline and erythromycin. Means for discovery of translation-targeted therapeutics for viral disease are discussed.
Figures

Similar articles
-
RNA-Binding Proteins as Regulators of Internal Initiation of Viral mRNA Translation.Viruses. 2022 Jan 19;14(2):188. doi: 10.3390/v14020188. Viruses. 2022. PMID: 35215780 Free PMC article. Review.
-
The Hinge Region of the Israeli Acute Paralysis Virus Internal Ribosome Entry Site Directs Ribosomal Positioning, Translational Activity, and Virus Infection.J Virol. 2022 Mar 9;96(5):e0133021. doi: 10.1128/JVI.01330-21. Epub 2022 Jan 12. J Virol. 2022. PMID: 35019716 Free PMC article.
-
Regulation of Ribosomal Proteins on Viral Infection.Cells. 2019 May 27;8(5):508. doi: 10.3390/cells8050508. Cells. 2019. PMID: 31137833 Free PMC article. Review.
-
Regulation of HIV-1 Gag-Pol Expression by Shiftless, an Inhibitor of Programmed -1 Ribosomal Frameshifting.Cell. 2019 Jan 24;176(3):625-635.e14. doi: 10.1016/j.cell.2018.12.030. Cell. 2019. PMID: 30682371 Free PMC article.
-
Viral subversion of the host protein synthesis machinery.Nat Rev Microbiol. 2011 Oct 17;9(12):860-75. doi: 10.1038/nrmicro2655. Nat Rev Microbiol. 2011. PMID: 22002165 Free PMC article. Review.
Cited by
-
Competing pathways control host resistance to virus via tRNA modification and programmed ribosomal frameshifting.Mol Syst Biol. 2012 Jan 31;8:567. doi: 10.1038/msb.2011.101. Mol Syst Biol. 2012. PMID: 22294093 Free PMC article.
-
Identification of structurally re-engineered rocaglates as inhibitors against hepatitis E virus replication.Antiviral Res. 2022 Aug;204:105359. doi: 10.1016/j.antiviral.2022.105359. Epub 2022 Jun 18. Antiviral Res. 2022. PMID: 35728703 Free PMC article.
-
Ribosomal frameshifting and transcriptional slippage: From genetic steganography and cryptography to adventitious use.Nucleic Acids Res. 2016 Sep 6;44(15):7007-78. doi: 10.1093/nar/gkw530. Epub 2016 Jul 19. Nucleic Acids Res. 2016. PMID: 27436286 Free PMC article. Review.
-
Translational control of viral gene expression in eukaryotes.Microbiol Mol Biol Rev. 2000 Jun;64(2):239-80. doi: 10.1128/MMBR.64.2.239-280.2000. Microbiol Mol Biol Rev. 2000. PMID: 10839817 Free PMC article. Review.
-
RNA targeting by small molecules: Binding of protoberberine, benzophenanthridine and aristolochia alkaloids to various RNA structures.J Biosci. 2012 Jul;37(3):539-52. doi: 10.1007/s12038-012-9217-3. J Biosci. 2012. PMID: 22750990 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
