Lock on/off disulfides identify the transmembrane signaling helix of the aspartate receptor
- PMID: 7592603
- PMCID: PMC2899691
- DOI: 10.1074/jbc.270.41.24043
Lock on/off disulfides identify the transmembrane signaling helix of the aspartate receptor
Abstract
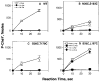
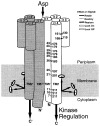
The aspartate receptor of the bacterial chemotaxis pathway regulates the autophosphorylation rate of a cytoplasmic histidine kinase in response to ligand binding. The transmembrane signal, which is transmitted from the periplasmic aspartate-binding domain to the cytoplasmic regulatory domain, is carried by an intramolecular conformational change within the homodimeric receptor structure. The present work uses engineered cysteines and disulfide bonds to probe the nature of this conformational change, focusing in particular on the role of the second transmembrane alpha-helix. Altogether 26 modifications, consisting of 13 cysteine pairs and the corresponding disulfide bonds, have been introduced into the contacts between the second transmembrane helix and adjacent helices. The effects of these modifications on the transmembrane signal have been quantified by in vitro assays which measure (i) ligand binding, (ii) receptor-mediated regulation of kinase activity, and (iii) receptor methylation. All three parameters are observed to be highly sensitive to perturbations of the second transmembrane helix. In particular, 13 of the 26 modifications (6 cysteine pairs and 7 disulfides) significantly increase or decrease aspartate affinity, while 15 of the 26 modifications (6 cysteine pairs and 10 disulfides) destroy transmembrane kinase regulation. Importantly, 3 of the perturbing disulfides are found to lock the receptor in the "on" or "off" signaling state by covalently constraining the second transmembrane helix, demonstrating that it is possible to use engineered disulfides to lock the signaling function of a receptor protein. A separate aspect of the study probes the thermal motions of the second transmembrane helix: 4 disulfides designed to trap large amplitude twisting motions are observed to disrupt function but form readily, suggesting that the helix is mobile. Together the results support a model in which the second transmembrane helix is a mobile signaling element responsible for communicating the transmembrane signal.
Figures
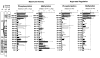
Similar articles
-
Transmembrane signaling by the aspartate receptor: engineered disulfides reveal static regions of the subunit interface.Biochemistry. 1995 Aug 1;34(30):9722-33. doi: 10.1021/bi00030a010. Biochemistry. 1995. PMID: 7626643 Free PMC article.
-
Cysteine and disulfide scanning reveals a regulatory alpha-helix in the cytoplasmic domain of the aspartate receptor.J Biol Chem. 1997 Dec 26;272(52):32878-88. doi: 10.1074/jbc.272.52.32878. J Biol Chem. 1997. PMID: 9407066 Free PMC article.
-
Engineered socket study of signaling through a four-helix bundle: evidence for a yin-yang mechanism in the kinase control module of the aspartate receptor.Biochemistry. 2009 Oct 6;48(39):9266-77. doi: 10.1021/bi901020d. Biochemistry. 2009. PMID: 19705835 Free PMC article.
-
Functional similarities among two-component sensors and methyl-accepting chemotaxis proteins suggest a role for linker region amphipathic helices in transmembrane signal transduction.Mol Microbiol. 1999 Sep;33(6):1093-102. doi: 10.1046/j.1365-2958.1999.01562.x. Mol Microbiol. 1999. PMID: 10510225 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Role of predicted transmembrane domains for type III translocation, pore formation, and signaling by the Yersinia pseudotuberculosis YopB protein.Infect Immun. 2005 Apr;73(4):2433-43. doi: 10.1128/IAI.73.4.2433-2443.2005. Infect Immun. 2005. PMID: 15784589 Free PMC article.
-
Evidence that both ligand binding and covalent adaptation drive a two-state equilibrium in the aspartate receptor signaling complex.J Gen Physiol. 2001 Dec;118(6):693-710. doi: 10.1085/jgp.118.6.693. J Gen Physiol. 2001. PMID: 11723162 Free PMC article.
-
Structure, function, and on-off switching of a core unit contact between CheA kinase and CheW adaptor protein in the bacterial chemosensory array: A disulfide mapping and mutagenesis study.Biochemistry. 2013 Nov 5;52(44):7753-65. doi: 10.1021/bi401159k. Epub 2013 Oct 22. Biochemistry. 2013. PMID: 24090207 Free PMC article.
-
Mutational analysis of a transmembrane segment in a bacterial chemoreceptor.J Bacteriol. 1996 Aug;178(15):4651-60. doi: 10.1128/jb.178.15.4651-4660.1996. J Bacteriol. 1996. PMID: 8755897 Free PMC article.
-
Thermosensing properties of mutant aspartate chemoreceptors with methyl-accepting sites replaced singly or multiply by alanine.J Bacteriol. 1997 Nov;179(21):6573-80. doi: 10.1128/jb.179.21.6573-6580.1997. J Bacteriol. 1997. PMID: 9352902 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

