An intelligent and cost-effective computer dosing system for individualizing FK506 therapy in transplantation and autoimmune disorders
- PMID: 7690046
- PMCID: PMC3016879
- DOI: 10.1002/j.1552-4604.1993.tb04711.x
An intelligent and cost-effective computer dosing system for individualizing FK506 therapy in transplantation and autoimmune disorders
Abstract
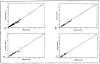
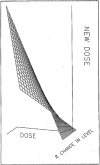
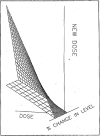
The accuracy and precision of an intelligent dosing system (IDS) for FK506 in predicting doses to achieve target drug levels has been prospectively evaluated in transplant and autoimmune patients. For dose individualization, the knowledge base is updated with patient-specific feedback including the current dose, drug level, and the new target level. The study population of 147 patients consisted of 97 transplant patients (liver and kidney) and 50 patients with autoimmune disorders. Patients in the transplant study group were entered sequentially and followed as a cohort. Patients in the autoimmune study group were randomly assigned to one of three predefined FK506 concentration windows (low, 0.1-.3; medium, 0.4-.7; and high, 0.8-1.3 ng/mL) as part of a concentration controlled clinical trial. Predictions of steady-state plasma drug levels were made throughout the clinical course of autoimmune patients and during the first 6 weeks post-transplant in liver and kidney recipients. FK506 concentration in plasma was measured by a monoclonal antibody based ELISA assay. Accuracy was computed as the mean prediction error (mpe). Precision was computed as the root mean squared prediction error (rmspe). The accuracy of the IDS in each study group was as follows: 0.016 ng/mL (liver), -0.034 ng/mL (kidney), and -0.022 ng/mL (autoimmune). Because the 95% confidence interval included zero in each case, the IDS showed no bias. The precision of the IDS in each study group was as follows: 0.133 ng mL (liver), 0.1903 ng/mL (kidney), and 0.1188 ng/mL (autoimmune). These results indicate that the FK506 IDS is both accurate and very precise (reproducible) in transplant and autoimmune patients.(ABSTRACT TRUNCATED AT 250 WORDS)
Figures

Comment in
-
FK506, artificial intelligence and pharmacoeconomics.J Clin Pharmacol. 1993 Jul;33(7):596-8. doi: 10.1002/j.1552-4604.1993.tb04710.x. J Clin Pharmacol. 1993. PMID: 7690045 No abstract available.
References
-
- Fung J, Abu-Elmagd K, Todo S, et al. FK506 in clinical organ transplantation. Clin Transplant. 1991;5:517–522.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

