Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation
- PMID: 7701323
- PMCID: PMC4242209
- DOI: 10.1126/science.7701323
Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation
Abstract
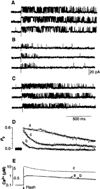
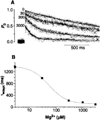
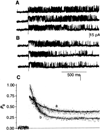
Channel adaptation is a fundamental feature of sarcoplasmic reticulum calcium release channels (called ryanodine receptors, RyRs). It permits successive increases in the intracellular concentration of calcium (Ca2+) to repeatedly but transiently activate channels. Adaptation of RyRs in the absence of magnesium (Mg2+) and adenosine triphosphate is an extremely slow process (taking seconds). Photorelease of Ca2+ from nitrophenyl-EGTA, a photolabile Ca2+ chelator, demonstrated that RyR adaptation is rapid (milliseconds) in canine heart muscle when physiological Mg2+ concentrations are present. Phosphorylation of the RyR by protein kinase A increased the responsiveness of the channel to Ca2+ and accelerated the kinetics of adaptation. These properties of the RyR from heart may also be relevant to other cells in which multiple agonist-dependent triggering events regulate cellular functions.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

