Molecular characterization of defective antigen processing in human prostate cancer
- PMID: 7707419
- PMCID: PMC2104544
- DOI: 10.1093/jnci/87.4.280
Molecular characterization of defective antigen processing in human prostate cancer
Abstract
Background: Gene-modified tumor cell vaccines have shown efficacy in animal models of malignancy, including prostate cancer. Class I major histocompatibility complex (MHC) assembly and function in the cellular targets of such therapies is pivotal in determining the efficacy of specific cytokine-secreting tumor vaccines.
Purpose: To help guide development of genetically engineered vaccine therapy for human prostate cancer, potential immune resistance pathways were evaluated by analysis of class I MHC assembly in prostate cancer cells.
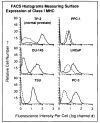
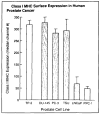
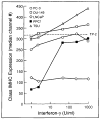
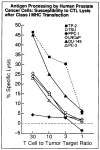
Method: Class I MHC assembly in metastasis-derived human prostate cancer cell lines (LNCaP, PPC-1, DU-145, PC-3, and TSU) and a normal prostate-derived cell line (TP-2) were characterized by phenotypic, molecular, and functional assays. Assembled class I MHC and antigen was measured by flow cytometry; mRNA levels of assembly components (class I MHC heavy chain, beta 2-microglobulin, and the antigen transporter gene product TAP-2) were determined; and antigen processing was measured with a chimeric reconstituted system using vaccinia vectors. Restoration of antigen processing was attempted by interferon gamma stimulation and by transfection with mouse class I MHC heavy-chain cDNA.
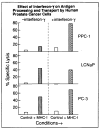
Results: Assembled class I MHC was underexpressed in two (LNCaP and PPC-1) of five prostate cancer cell lines compared with normal prostate-derived controls. PPC-1 cells underexpressed TAP-2 mRNA despite abundant class I MHC and beta 2-microglobulin message. Induction of TAP-2 by interferon gamma indicated that coding sequences for TAP-2 message were present in PPC-1. Resistance to cytotoxic T lymphocytes (CTL) lysis showed a functional defect in antigen transport by PPC-1 cells; reversal of the molecular defect with interferon gamma led to restoration of functional antigen processing. In contrast, LNCaP cells had competent antigen transport but deficient class I MHC heavy-chain function despite abundant class I MHC RNA; though refractory to stimulation by interferon gamma, this defect responded to transfection of class I MHC heavy-chain cDNA.
Conclusions: Metastatic prostate cancer cells can escape T-cell recognition via divergent mechanisms of defective class I MHC assembly. The specific underexpression of TAP-2 gene product in PPC-1 cells contrasts with prior studies of TAP gene underexpression in lung cancer (which concurrently underexpressed class I MHC heavy chain) and provides evidence for a regulatory pathway controlling TAP-2 gene expression in human cancers that may not affect class I MHC heavy-chain expression.
Implications: In clinical application of gene therapy for prostate cancer, these findings provide a rationale for focusing on strategies that can circumvent sole reliance on class I MHC-mediated tumor cell recognition by CTL.
Figures

Comment in
-
Genetic instability and tumor cell variation: implications for immunotherapy.J Natl Cancer Inst. 1995 Feb 15;87(4):241-3. doi: 10.1093/jnci/87.4.241. J Natl Cancer Inst. 1995. PMID: 7707413 No abstract available.
References
-
- Walsh PC. Prostate cancer kills: a strategy to reduce deaths. Urology. 1994;44:463–466. - PubMed
-
- Golumbek PT, Lazenby AJ, Levitsky HI, et al. Treatment of established renal cell cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–716. - PubMed
-
- Trojan J, Johnson TR, Rudin SD, et al. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

