IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases
- PMID: 7730632
- PMCID: PMC2041892
IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases
Abstract
Neoplastic cells are generally poor immunogens. Transfection of the murine tumor CT-26 with beta-galactosidase (beta-gal), a protein from Escherichia coli, did not alter its growth rate in vivo, or its lethality, and did not elicit a measurable anti-beta-gal immune response. Immunization with beta-gal-expressing recombinant vaccinia viruses (rVV) elicited specific anti-beta-gal cytolytic T lymphocytes, but rVV-beta-gal was only marginally therapeutic when given to tumor-bearing mice. With the aim of expanding the immune response against beta-gal, used here as a model tumor Ag, we gave mice exogenous IL-2 starting 12 h after the poxvirus. The therapeutic effectiveness of the combination of poxvirus and IL-2 was far greater than either of these treatments alone. When the cDNA for IL-2 was inserted into the viral genome of the rVV construct to make a double recombinant (drVV), antitumor activity was further augmented. One mechanism of action may be the enhanced activation or expansion of cytotoxic T cells, because a marked increase in primary cytotoxic responses against vaccinia determinants was observed. Interestingly, other cytokines (mGM-CSF, mTNF-alpha, and mIFN-gamma) inserted into the rVV genome did not modify the efficacy of the rVV constructs. The increase in specific CTL responses against beta-gal by drVV expressing the tumor-associated Ags (TAA) and IL-2 was more pronounced in mice bearing the lacZ-transduced tumor than in those bearing the parental cell line, suggesting that the TAA presented by growing tumor cells can either pre-activate or otherwise amplify the immune response induced by the rVV. Unfortunately, in several long-term surviving mice, tumor recurred that no longer expressed beta-gal. These results indicate that treatment of disseminated tumors by using recombinant viruses expressing TAA can be enhanced by IL-2 provided exogenously, or encoded within the recombinant virus.
Figures

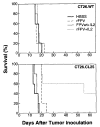


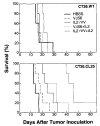


References
-
- Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
