Synthesis and maturation of viral transcripts in herpes simplex virus type 1 infected HeLa cells: the role of interchromatin granules
- PMID: 7734948
- PMCID: PMC6134381
Synthesis and maturation of viral transcripts in herpes simplex virus type 1 infected HeLa cells: the role of interchromatin granules
Abstract
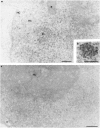
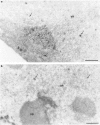
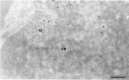
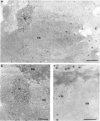
The response of the cellular RNA processing machinery to herpes simplex virus type 1 (HSV-1) infection was studied at the ultrastructural level in HeLa cells and compared to the distribution of RNA polymerase II molecules and viral RNA. Immunogold labeling of RNA polymerase II molecules revealed that viral genome transcription was restricted to filaments in an intranuclear, virus-induced region. This region also contained viral RNAs as revealed by in situ hybridization of two biotinylated viral DNA probes: a probe encompassing a limited portion of the viral genome (the F fragment) and a probe for the total genome. In addition, the latter probe revealed large amounts of viral RNA within the clusters of interchromatin granules, intranuclear structures of normal cells that became enlarged during HSV-1 infection. Components of spliceosomes were localized by in situ hybridization with biotinylated U1 and U2 DNA probes. The large viral region contained only traces of U1 and U2 RNAs, probably because of the low frequency of splices of viral transcripts. The clusters of interchromatin granules, however, accumulated U1 and U2 RNAs with the same frequency as in noninfected cells. Poly(A) RNA was detected by in situ hybridization of a biotinylated poly(dT) probe. Some was present over the filaments of the virus-induced region but most was accumulated in the clusters of interchromatin granules. Our data suggest, therefore, that the clusters of interchromatin granules, in addition to their involvement in spliceosome component assembly, might also be a transient storage site for some families of viral mRNA, possibly a sorting site that regulates their migration.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
