Conditional root expansion mutants of Arabidopsis
- PMID: 7743935
- PMCID: PMC4353850
- DOI: 10.1242/dev.121.4.1237
Conditional root expansion mutants of Arabidopsis
Abstract
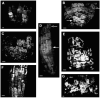
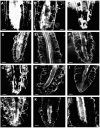
Regulation of cell expansion is essential to the formation of plant organs. We have characterized 21 mutations, representing six loci, that cause abnormal cell expansion in the root of Arabidopsis thaliana. The phenotype of these mutants is conditional upon the rate of root growth. Calculation of cell volumes indicated that the mutations resulted in defects in either the orientation or the extent of expansion or in both. Analysis of cortical microtubules in the mutants suggested that a shift in the orientation of cell expansion may not be dependent on a change in the orientation of the microtubules. Double mutant combinations resulted in loss of the conditional phenotype suggesting that the genes may act in a similar pathway or encode partially redundant functions.
Figures






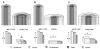
References
-
- Aeschbacher RA, Hauser M-T, Feldmann KA, Benfey PN. The SABRE gene is required for normal cell expansion in Arabidopsis. Genes Dev. 1995 in press. - PubMed
-
- Baluska F, Brailsford RW, Hauskrecht M, Jackson MB, Barlow PW. Cellular dimorphism in the maize root cortex: involvement of microtubules, ethylene and gibberellin in the differentiation of cellular behaviour in postmitotic growth zones. Acta Bot. 1993;106:394–403.
-
- Baskin TI, Betzner AS, Hoggart R, Cork A, Williamson RE. Root morphology mutants in Arabidopsis thaliana. Aust. J. Plant Physiol. 1992;19:427–438.
-
- Baskin TI, Wilson JE, Cork A, Williamson RE. Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol. 1994;35:935–942. - PubMed
-
- Baskin TI, Williamson RE. Ethylene, microtubules and root morphology in wild-type and mutant Arabidopsis seedlings. Curr. Top. Pl. Biochem. Physiol. 1992;11:118–130.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

