Role of homeodomain protein binding region in the expression of Drosophila proliferating cell nuclear antigen gene: analysis with transgenic flies
- PMID: 7787411
- PMCID: PMC6134388
Role of homeodomain protein binding region in the expression of Drosophila proliferating cell nuclear antigen gene: analysis with transgenic flies
Abstract
The regulatory region of Drosophila proliferating cell nuclear antigen (PCNA) gene consists of a promoter region (-168 to +24 with respect to the transcription initiation site) and an upstream region containing three homeodomain protein binding sites (HDB) (-357 to -165). The PCNA gene regulatory regions with HDB (-607 to +137) or without HDB (-168 to +137) were fused with the lacZ and transgenic flies were established by P-element-mediated transformation. Male transgenic flies were crossed with wild-type females, and zygotic expression of the lacZ was monitored by quantitative beta-galactosidase assay, at various stages of development. Expression of the lacZ was high in embryos, first and second instar larvae, and adult females, and low at other stages of development. Only a marginal difference in expression was observed between flies carrying the homeodomain protein binding region and those not carrying it. Spatial pattern of the lacZ expression in the embryo visualized by immunostaining with the anti-lacZ antibody was similar to the distribution of the endogenous PCNA protein. Here, too, only a marginal difference was observed between transgenic flies carrying two different constructs of the PCNA lacZ. In genetic crossing experiments of transgenic flies with those carrying mutation in homeobox genes, no significant change in the lacZ expression pattern was observed. However, when male transgenic flies were crossed with female flies homozygous for a torso gain-of-function allele, repression of the lacZ expression was observed in the central region of the embryo. Because these local changes in the lacZ expression depend on the homeodomain protein binding region, unidentified homeodomain proteins are probably involved. Our results suggest that the promoter region is practically sufficient for expression of the PCNA gene and that the homeodomain protein binding region functions as a silencer when torso is activated ectopically.
Figures

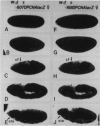

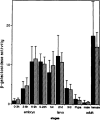
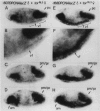
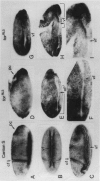
Similar articles
-
Roles of multiple promoter elements of the proliferating cell nuclear antigen gene during Drosophila development.Genes Cells. 1996 Jan;1(1):47-58. doi: 10.1046/j.1365-2443.1996.03003.x. Genes Cells. 1996. PMID: 9078366
-
Repression of the Drosophila proliferating-cell nuclear antigen gene promoter by zerknüllt protein.Mol Cell Biol. 1991 Oct;11(10):4909-17. doi: 10.1128/mcb.11.10.4909-4917.1991. Mol Cell Biol. 1991. PMID: 1681423 Free PMC article.
-
A binding site for the transcription factor Grainyhead/Nuclear transcription factor-1 contributes to regulation of the Drosophila proliferating cell nuclear antigen gene promoter.J Biol Chem. 1999 Dec 3;274(49):35080-8. doi: 10.1074/jbc.274.49.35080. J Biol Chem. 1999. PMID: 10574988
-
Identification of CFDD (common regulatory factor for DNA replication and DREF genes) and role of its binding site in regulation of the proliferating cell nuclear antigen gene promoter.J Biol Chem. 1997 Sep 5;272(36):22848-58. doi: 10.1074/jbc.272.36.22848. J Biol Chem. 1997. PMID: 9278447
-
Distribution of PCNA during postblastoderm cell division cycles in the Drosophila melanogaster embryo: effect of a string- mutation.Cell Struct Funct. 1995 Feb;20(1):47-57. doi: 10.1247/csf.20.47. Cell Struct Funct. 1995. PMID: 7796467
Cited by
-
Characterization of dRFX2, a novel RFX family protein in Drosophila.Nucleic Acids Res. 2004 Oct 19;32(18):5636-48. doi: 10.1093/nar/gkh895. Print 2004. Nucleic Acids Res. 2004. PMID: 15494451 Free PMC article.
-
E2F-dependent transcription of the raf proto-oncogene during Drosophila development.Nucleic Acids Res. 2001 Apr 15;29(8):1808-14. doi: 10.1093/nar/29.8.1808. Nucleic Acids Res. 2001. PMID: 11292854 Free PMC article.
References
-
- Campos-Ortega J. A. and Hartenstein V. (1985), in The Embryonic Development of Drosophila melanogaster, Springer-Verlag, Berlin, pp. 210–217.
-
- Casanova J. and Struhl G. (1989), Genes Dev 3, 2025–2038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
