In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Implications for the establishment and maintenance of donor cell chimerism following liver transplantation
- PMID: 7878760
- PMCID: PMC3091389
- DOI: 10.1097/00007890-199502270-00019
In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Implications for the establishment and maintenance of donor cell chimerism following liver transplantation
Abstract
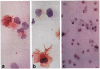
Dendritic cell (DC) progenitors were propagated in liquid culture from nonparenchymal cells resident in normal mouse (B10.BR; H-2k, I-E+) liver in response to granulocyte-macrophage colony stimulating factor (GM-CSF). The liver-derived DC progenitors were MHC class II-/dim and did not express counter receptors for CTLA-4, a structural homologue of the T cell activation molecule CD28. Following subcutaneous or intravenous injection, these liver-derived cells migrated to T cell-dependent areas of lymph nodes and spleen of unmodified, allogeneic (B10; H-2b; I-E-) recipients, where they were identified 1-5 days, and 1 and 2 months after injection by their strong surface expression of donor MHC class II (I-Ek) and their dendritic morphology. Maximal numbers of liver-derived DC in the spleen were recorded 5 days after injection. Both clusters of strongly donor MHC class II+ cells--and (more rarely) dividing cells--could also be identified, suggesting cell replication in situ. Using the same techniques employed to generate DC progenitors from normal liver, GM-CSF-stimulated cells were propagated for 10 days from the bone marrow and spleen of nonimmunosuppressed mice sacrificed 14 days after orthotopic liver transplantation (B10;H-2b-->C3H;H-2k). Immunocytochemical staining for recipient and donor MHC class II phenotype revealed the growth both of host cells with DC characteristics, and of cells expressing donor alloantigens (I-Ab). These results are consistent with the growth, in response to GM-CSF, of donor-derived DC from progenitors seeded from the liver allograft to recipient lymphoid tissue. The functional activity of the progenitors of chimeric DC and the possible role of these cells in the establishment and maintenance of donor-specific tolerance following liver transplantation remain to be determined.
Figures
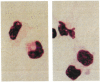

 ] using naive, B10 (I-E−) splenic T cells as responders. The nonadherent, low-buoyant-density cells were harvested from 10-day GM-CSF-stimulated cultures and set up at various concentrations, with 4×105 responder T cells. Cultures were maintained for 72 hr; [3H]TdR was added 18 hr before harvesting. The MLR-stimulatory activity of freshly-isolated allogeneic (B10.BR) liver NPC [
] using naive, B10 (I-E−) splenic T cells as responders. The nonadherent, low-buoyant-density cells were harvested from 10-day GM-CSF-stimulated cultures and set up at various concentrations, with 4×105 responder T cells. Cultures were maintained for 72 hr; [3H]TdR was added 18 hr before harvesting. The MLR-stimulatory activity of freshly-isolated allogeneic (B10.BR) liver NPC [ ] and spleen cells [
] and spleen cells [ ] and of syngeneic (B10; [
] and of syngeneic (B10; [ ]) spleen cells is also shown. The results are expressed as mean counts per minute (cpm) ± 1 SD and are representative of 3 separate experiments.
]) spleen cells is also shown. The results are expressed as mean counts per minute (cpm) ± 1 SD and are representative of 3 separate experiments.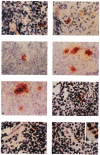

References
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
