Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate
- PMID: 7993631
- PMCID: PMC2211371
- DOI: 10.1016/0896-6273(94)90425-1
Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate
Abstract
The effect of protein kinase C (PKC) on rapid N-type inactivation of K+ channels has not been reported previously. We found that PKC specifically eliminates rapid inactivation of a cloned human A-type K+ channel (hKv3.4), converting this channel from a rapidly inactivating A type to a noninactivating delayed rectifier type. Biochemical analysis showed that the N-terminal domain of hKv3.4 is phosphorylated in vitro by PKC, and mutagenesis experiments revealed that two serines within the inactivation gate at the N-terminus are sites of direct PKC action. Moreover, mutating one of these serines to aspartic acid mimics the action of PKC. Serine phosphorylation may thus prevent rapid inactivation by shielding basic residues known to be critical to the function of the inactivation gate. The regulatory mechanism reported here may have substantial effects on signal coding in the nervous system.
Figures
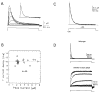



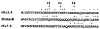

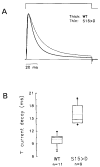
References
-
- Busch AE, Varnum MD, North RA, Adelman JP. An amino acid mutation in a potassium channel that prevents inhibition by protein kinase C. Science. 1992;255:1705–1707. - PubMed
-
- Chung SK, Reinhart PH, Martin BL, Brautigan D, Levitan IB. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991;253:560–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

