Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes
- PMID: 8001117
- PMCID: PMC3082463
- DOI: 10.1016/0092-8674(94)90068-x
Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes
Abstract
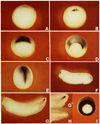
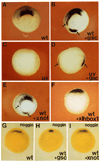
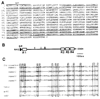
A Xenopus gene whose expression can be activated by the organizer-specific homeobox genes goosecoid and Xnot2 was isolated by differential screening. The chordin gene encodes a novel protein of 941 amino acids that has a signal sequence and four Cys-rich domains. The expression of chordin starts in Spemann's organizer subsequent to that of goosecoid, and its induction by activin requires de novo protein synthesis. Microinjection of chordin mRNA induces twinned axes and can completely rescue axial development in ventralized embryos. This molecule is a potent dorsalizing factor that is expressed at the right time and in the right place to regulate cell-cell interactions in the organizing centers of head, trunk, and tail development.
Figures



References
-
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. - PubMed
-
- Blumberg B, Wright CVE, De Robertis EM, Cho KWY. Organizer-specific homeogenes in Xenopus laevis embryos. Science. 1991;253:194–196. - PubMed
-
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992;6:3290–3299. - PubMed
-
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

