Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells
- PMID: 8073517
- PMCID: PMC3091356
- DOI: 10.1097/00007890-199408270-00015
Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells
Abstract
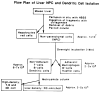
Donor liver-derived dendritic cells (DC) have recently been identified within various lymphoid and nonlymphoid tissues of organ allograft recipients, including nonimmunosuppressed mice transplanted with and permanently accepting major histocompatibility complex (MHC)-disparate hepatic allografts. These findings have raised questions about the basis of the tolerogenicity of the liver--and, in particular, about the properties of liver-derived DC. To study further the structure, immunophenotype and allostimulatory activity of leukocytes resident in normal mouse (B10.BR;H-2k, I-Ek) liver, a procedure was developed to maximize the yield of viable, nonparenchymal cells (NPC) obtained following collagenase digestion of perfused liver fragments and density centrifugation (Percoll). These cells comprised populations expressing lymphoid and myeloid cell surface antigens. As compared with spleen cells, they proved good allostimulators of naive (B10; H-2b, I-E-) splenic T cells when tested in primary mixed leukocyte reactions (MLR). After overnight (18-hr) incubation of the NPC, enrichment for transiently adherent, low-density (LD) cells on metrizamide gradients permitted the recovery of low numbers of cells (approx. 2-5 x 10(5) per liver), many of which displayed distinct DC morphology. Flow cytometric analysis revealed that these cells were CD3-, CD4-, CD8-, and B220-, but strongly expressed CD45 (leukocyte-common antigen), and mild-to-moderate levels of CD11b, heat-stable antigen, and CD44. The cells also expressed moderate intensity of NLDC 145 but not 33D1, DC restricted markers which have been shown to be differentially expressed on mouse DC isolated from various organs. This DC-enriched population was more strongly MHC class II(I-Ek)+ than NPC, as determined by immunocytochemistry and flow cytometry and exhibited much more potent allostimulatory activity for naive T cells. These findings demonstrate that freshly isolated murine liver NPC, and perhaps their counterparts in situ, exhibit allostimulatory activity that is enhanced in the non-adherent, low-density (DC-enriched) fraction after overnight culture. They further suggest that the maturation of liver DC may play a key role in determining the immunogenicity and or tolerogenicity of hepatic allografts.
Figures







References
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. - PubMed
-
- Knight SC. Dendritic cells. In: Lachmann PJ, Peters K, Rosen FS, Walport MJ, editors. Clinical aspects of immunology. Blackwell; Oxford: 1993. p. 481.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
