Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus
- PMID: 8099201
- PMCID: PMC2096733
- DOI: 10.1038/363451a0
Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus
Abstract
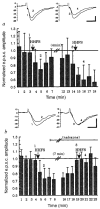
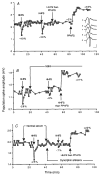
Although anatomical and neurochemical studies suggest that endogenous opioids act as neurotransmitters, their roles in normal and pathophysiological regulation of synaptic transmission are not defined. Here we examine the actions of prodynorphin-derived opioid peptides in the guinea-pig hippocampus and show that physiological stimulation of the dynorphin-containing dentate granule cells can release endogenous dynorphins, which then activate kappa 1 opioid receptors present in the molecular layer of the dentate gyrus. Activation of kappa 1 receptors by either pharmacologically applied agonist or endogenously released peptide reduces excitatory transmission in the dentate gyrus, as shown by a reduction in the excitatory postsynaptic currents evoked by stimulation of the perforant path, a principal excitatory afferent. In addition, released dynorphin peptides were found to block the induction of long-term potentiation (LTP) at the granule cell-perforant path synapse. The results indicate that endogenous dynorphins function in this hippocampal circuit as retrograde, inhibitory neurotransmitters.
Figures
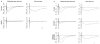
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

