Comparison of four basic models of indirect pharmacodynamic responses
- PMID: 8133465
- PMCID: PMC4207304
- DOI: 10.1007/BF01061691
Comparison of four basic models of indirect pharmacodynamic responses
Abstract
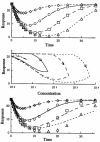
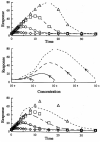
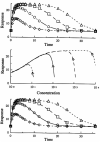
Four basic models for characterizing indirect pharmacodynamic responses after drug administration have been developed and compared. The models are based on drug effects (inhibition or stimulation) on the factors controlling either the input or the dissipation of drug response. Pharmacokinetic parameters of methylprednisolone were used to generate plasma concentration and response-time profiles using computer simulations. It was found that the responses produced showed a slow onset and a slow return to baseline. The time of maximal response was dependent on the model and dose. In each case, hysteresis plots showed that drug concentrations preceded the response. When the responses were fitted with pharmacodynamic models based on distribution to a hypothetical effect compartment, the resulting parameters were dose-dependent and inferred biological implausibility. Indirect response models must be treated as distinct from conventional pharmacodynamic models which assume direct action of drugs. The assumptions, equations, and data patterns for the four basic indirect response models provide a starting point for evaluation of pharmacologic effects where the site of action precedes or follows the measured response variable.
Figures

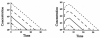


References
-
- Holford NHG, Sheiner LB. Understanding the dose-effect relationship: Clinical application of pharmacokinetic-pharmacodynamic models. cin Pharmacokin. 1981;6:429–453. - PubMed
-
- Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371. - PubMed
-
- Verotta D, Beal SL, Sheiner LB. Semiparametric approach to pharmacokinetic-pharmacodynamic data. Am. J. Physiol. 1989;256:R1005–R1010. - PubMed
-
- Holford NHG, Sheiner LB. Pharmacokinetic and pharmacodynamic modeling in vivo. CRC Crit. Rev. Bioeng. 1981;5:273–322. - PubMed
-
- Lalonde RL. Applied Pharmacokinetics. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Therapeutics. 3rd Vancouver: 1992. pp. 4–33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
