Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms
- PMID: 8223267
- PMCID: PMC2758228
- DOI: 10.1242/dev.118.2.377
Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms
Abstract
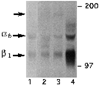
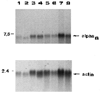
We have recently shown that the laminin-binding integrin receptor, alpha 6 beta 1, is prominently expressed in the developing chick retina, and its expression and activity are regulated during development on both retinal ganglion cells and other neural retinal cells. In the present study, we show that antibodies specific for the extracellular portion of the chick alpha 6 subunit dramatically inhibit interactions in vitro between embryonic day 6 neural retinal cells and laminin, showing that alpha 6 beta 1 functions as an important laminin receptor on developing retinal neurons. In previous work, we showed that alpha 6 mRNA levels on retinal ganglion cells decrease dramatically after E6 during the period that RGC axons innervate the optic tectum. In the present study, we show decreases in alpha 6 mRNA are not prevented by ablation of the optic tectum, indicating that tectal contact is not the major cause of this decrease. Within the embryonic retina, the alpha 6 subunit is codistributed, in part, with laminin, suggesting that it functions as a laminin receptor during retina development in vivo. Furthermore, two isoforms of the alpha 6 protein with distinct cytoplasmic domains generated by differential splicing have quite different distribution patterns in the retina, suggesting that these two isoforms may have different functions during retinal development.
Figures






References
-
- Adler R, Jerdan J, Hewitt AT. Responses of cultured neural retinal cells to substratum-bound laminin and other extracellular matrix molecules. Dev. Biol. 1985;112:100–114. - PubMed
-
- Bodary SC, Napier MA, McLean JW. Expression of recombinant platelet glycoprotein IIb/IIIa results in a functional fibrinogen-binding complex. J. Biol. Chem. 1989;264:18859–18862. - PubMed
-
- Bottenstein JE, Skaper SD, Varon S, Sato J. Selective survival of neurons from chick embryo sensory ganglionic dissociates by use of a defined, serum-free medium. Exp. Cell Res. 1980;125:183–190. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

