Identification of a 34 kDa protein specific to synaptic vesicles
- PMID: 8358632
- PMCID: PMC4702249
- DOI: 10.1016/0006-8993(93)90197-u
Identification of a 34 kDa protein specific to synaptic vesicles
Abstract
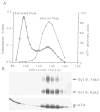
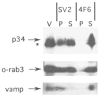
In this study, we used synaptic vesicles purified from the electric organ of marine electric rays to search for novel molecules which have important functions in synaptic transmission. Proteins that copurified with synaptic vesicles were used to immunize rats, and the resulting antisera were then used to further characterize the vesicle proteins. One of the antisera recognizes a protein of 34 kDa, p34, that has several characteristics which suggest it is a synaptic vesicle specific protein: (1) it copurifies exclusively with the synaptic vesicle peak during permeation chromatography on a controlled pore glass beads column, (2) it can be immunoprecipitated with intact synaptic vesicles and (3) it is specifically localized to the nervous system. The results suggest that p34 is a synaptic vesicle specific protein with a widespread distribution in the nervous system.
Figures
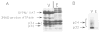
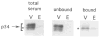

References
-
- Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. - PubMed
-
- Carlson SS, Wagner JA, Kelly RB. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978;17:1188–1199. - PubMed
-
- Cowan D, Linial M, Scheller RH. Torpedo synaptophysin: evolution of a synaptic vesicle protein. Brain Res. 1990;509:1–7. - PubMed
-
- Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

