Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance
- PMID: 8514264
- PMCID: PMC2964270
Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance
Abstract
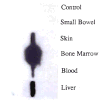
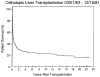
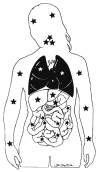
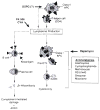
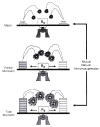
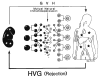
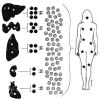
Improvements in the prevention or control of rejection of the kidney and liver have been largely interchangeable (1, 2) and then applicable, with very little modification, to thoracic and other organs. However, the mechanism by which anti rejection treatment permits any of these grafts to be “accepted” has been an immunological enigma (3, 4). We have proposed recently that the exchange of migratory leukocytes between the transplant and the recipient with consequent long-term cellular chimerism in both is the basis for acceptance of all whole-organ allografts and xenografts (5). Although such chimerism was demonstrated only a few months ago, the observations have increased our insight into transplantation immunology and have encouraged the development of alternative therapeutic strategies (6).
Figures























Comment in
-
Donor-derived chimerism in recipients of organ transplants.Hepatology. 1993 Jun;17(6):1153-6. Hepatology. 1993. PMID: 8514265 No abstract available.
References
-
- Starzl TE. Experience in renal transplantation. Philadelphia: WB Saunders Co; 1964. pp. 1–383.
-
- Starzl TE, Putnam CW. Experience in hepatic transplantation. Philadelphia: WB Saunders Co; 1969. pp. 1–553.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
