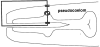
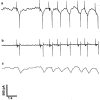
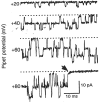
Electrophysiological methods
Figures

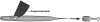



References
-
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. 1976;B275:299–325. - PubMed
-
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990;253:263–270. - PubMed
-
- Brown KT, Flaming DG. Advanced Micropipette Techniques for Cell Physiology. New York; Wiley: 1986.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
