Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes
- PMID: 8545887
- PMCID: PMC3000171
- DOI: 10.1097/00007890-199560120-00028
Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes
Abstract
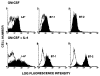
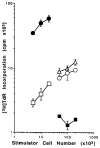
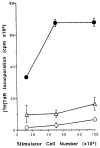
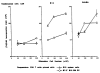
The functional maturation of dendritic cells (DC) and other antigen-presenting cells is believed to reflect the upregulation of cell surface major histocompatibility complex (MHC) class II and other T cell co-stimulatory molecules, especially the CD28 ligands B7-1 (CD80) and B7-2 (CD86). In this study, we propagated cells exhibiting characteristics of DC precursors from the bone marrow (BM) of B10 mice (H-2b; I-A+) in response to granulocyte-macrophage colony stimulating factor (GM-CSF). The methods used were similar to those employed previously to propagate DC progenitors from normal mouse liver. Cells expressing DC lineage markers (NLDC 145+, 33D1+, N418+) harvested from 8-10-day GM-CSF stimulated BM cell cultures were CD45+, heat-stable antigen+, CD54+, CD44+, MHC class II+, B7-1dim but B7-2- (costimulatory molecule-deficient). Supplementation of cultures with interleukin-4 (IL-4) in addition to GM-CSF however, resulted in marked upregulation of MHC class II and B7-2 expression. These latter cells exhibited potent allostimulatory activity in primary mixed leukocyte cultures. In contrast, the cells stimulated with GM-CSF alone were relatively weak stimulators and induced alloantigen-specific hyporesponsiveness in allogeneic T cells (C3H; H-2k; I-E+) detected upon restimulation in secondary MLR. This was associated with blockade of IL-2 production. Reactivity to third-party stimulators was intact. The hyporesponsiveness induced by the GM-CSF stimulated, costimulatory molecule-deficient cells was prevented by incorporation of anti-CD28 monoclonal antibody in the primary MLR and was reversed by addition of IL-2 to restimulated T cells. The findings show that MHC class II+ B7-2- cells with a DC precursor phenotype can induce alloantigen-specific hyporesponsiveness in vitro. Under the appropriate conditions, such costimulatory molecule-deficient cells could contribute to the induction of donor-specific unresponsiveness in vivo.
Figures

Similar articles
-
Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86-) prolong cardiac allograft survival in nonimmunosuppressed recipients.Transplantation. 1996 Sep 15;62(5):659-65. doi: 10.1097/00007890-199609150-00021. Transplantation. 1996. PMID: 8830833 Free PMC article.
-
Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen.J Exp Med. 1994 Jun 1;179(6):1823-34. doi: 10.1084/jem.179.6.1823. J Exp Med. 1994. PMID: 8195710 Free PMC article.
-
Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients.Transplantation. 1995 Dec 27;60(12):1555-9. doi: 10.1097/00007890-199560120-00030. Transplantation. 1995. PMID: 8545889 Free PMC article.
-
Microchimerism, dendritic cell progenitors and transplantation tolerance.Stem Cells. 1995 Nov;13(6):622-39. doi: 10.1002/stem.5530130607. Stem Cells. 1995. PMID: 8590864 Free PMC article. Review.
-
MicroRNAs affect dendritic cell function and phenotype.Immunology. 2015 Feb;144(2):197-205. doi: 10.1111/imm.12390. Immunology. 2015. PMID: 25244106 Free PMC article. Review.
Cited by
-
Blocking of the B7-CD28 pathway increases apoptosis induced in activated T cells by in vitro-generated CD95L (FasL) positive dendritic cells.Transplant Proc. 1997 Feb-Mar;29(1-2):1094-5. doi: 10.1016/s0041-1345(96)00424-1. Transplant Proc. 1997. PMID: 9123216 Free PMC article. No abstract available.
-
Contrasting effects of myeloid dendritic cells transduced with an adenoviral vector encoding interleukin-10 on organ allograft and tumour rejection.Immunology. 2000 Oct;101(2):233-41. doi: 10.1046/j.1365-2567.2000.00096.x. Immunology. 2000. PMID: 11012777 Free PMC article.
-
CD40-deficient dendritic cells producing interleukin-10, but not interleukin-12, induce T-cell hyporesponsiveness in vitro and prevent acute allograft rejection.Immunology. 1999 Oct;98(2):159-70. doi: 10.1046/j.1365-2567.1999.00863.x. Immunology. 1999. PMID: 10540214 Free PMC article.
-
Passive transfer of flt-3L-derived dendritic cells delays diabetes development in NOD mice and associates with early production of interleukin (IL)-4 and IL-10 in the spleen of recipient mice.Clin Exp Immunol. 2003 Dec;134(3):388-95. doi: 10.1111/j.1365-2249.2003.02308.x. Clin Exp Immunol. 2003. PMID: 14632742 Free PMC article.
-
Induction of peripheral tolerance by local delivery of dendritic cell progenitors to cardiac allografts in a murine heterotopic heart transplantation model.Gen Thorac Cardiovasc Surg. 2007 Aug;55(8):307-14. doi: 10.1007/s11748-007-0133-7. Gen Thorac Cardiovasc Surg. 2007. PMID: 17867275
References
-
- Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7–1 and B7–2) expression on murine dendritic cells. J Immunol. 1994;152:5208. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. - PubMed
-
- Austyn JM. The initiation of immune responses and allograft rejection. In: Rose ML, Yacoub MH, editors. Immunology of heart and lung transplantation. Little, Brown; Boston: 1993. p. 22.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
