Tetrahydrobenzothiophenone derivatives as a novel class of adenosine receptor antagonists
- PMID: 8558508
- PMCID: PMC10794914
- DOI: 10.1021/jm9504823
Tetrahydrobenzothiophenone derivatives as a novel class of adenosine receptor antagonists
Abstract
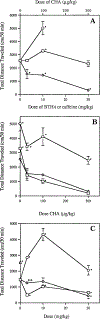
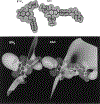
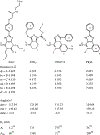
A novel class of non-nitrogen-containing heterocycles, the tetrahydrobenzothiophenones, was found to bind to adenosine receptors as antagonists in the micromolar range. Affinity was determined in radioligand-binding assays at rat brain A1 and A2a receptors. A structure-activity analysis indicated that a 3-thioether group is favored and affinity at A2a, but not at A1, receptors is highly dependent on this thioether substituent. A carboxylic acid-derived substituent is required at the 1-position of the thiophene ring, with esters being more potent in binding at A1 receptors than the corresponding carboxyl hydrazide or carboxylic acid derivatives. The methyl (15) and ethyl (16) esters are about equipotent at A1 but not at A2a receptors. A 4-keto group on the saturated ring is favored for receptor affinity. Dimethyl substitution at the 6-position of the saturated ring is allowed. One of the most potent derivatives was the nonselective compound ethyl 3-(benzylthio)-4-oxo-4,5,6,7-tetrahydrobenzo[c] thiophene-1-carboxylate (BTH4, 7; Figure 1), which antagonized adenosine agonist-induced inhibition of adenylyl cyclase in rat adipocyte membranes with a KB value of 1.62 +/- 0.73 microM and adenosine agonist-induced stimulation of adenylyl cyclase in pheochromocytoma cell membranes with a KB value of 9.19 +/- 0.98 microM. Displacement of radioligand binding by BTH4 (7) at cloned human A3 receptors was negligible but one slightly A3 selective compound (11, 3.9-fold over A1 and >7.5-fold over A2a) was found. A 1-methylpropyl thioether (17) was 29-fold selective for A1 and A2a receptors. BTH4 (7) alone, at 10 mg/kg, stimulated locomotor activity in mice but paradoxically acted, under certain circumstances, synergistically with an A1 selective agonist to depress locomotor activity. A pharmacophore model relating structural features of xanthine and non-xanthine adenosine antagonists to BTH4 (7) suggests a high degree of similarity in electrostatic surfaces, assuming that the thiophene ring superimposes the region of the uracil ring of xanthines.
Figures
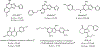

References
-
- Olsson RA; Pearson JD Cardiovascular purinoceptors. Pharmacol. Rev. 1990, 3, 761–845. - PubMed
-
- Hillaire-Buys D; Bertrand G; Gross R; Loubatières-Mariani MM Evidence for an inhibitory A1 subtype adenosine receptor on pancreatic insulin-secreting cells. Eur. J. Pharmacol. 1987, 136, 109–112. - PubMed
-
- Fredholm BB; Dunwiddie TV How does adenosine inhibit transmitter release? Trends Pharmacol. Sci. 1988, 9, 130–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

