Novel flow-cytometric method for separating cell types in differentiated F9 embryoid bodies
- PMID: 8582234
- PMCID: PMC4729370
- DOI: 10.1002/cyto.990210206
Novel flow-cytometric method for separating cell types in differentiated F9 embryoid bodies
Abstract
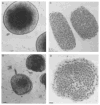
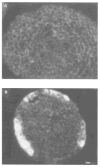
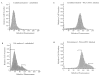
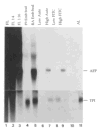
The differentiation of F9 teratocarcinoma cells mimics the formation of a mouse embryonic tissue, the primitive endoderm. In vitro, small aggregates of F9 cells, termed embryoid bodies, differentiate in response to retinoic acid and develop a surface epithelium that is characterized by the production of alpha-fetoprotein. In the present study, cellular autofluorescence profiles obtained by fluorescence-activated embryoid bodies were composed of a single type of cell. In contrast, retinoic acid-induced embryoid bodies were composed of two cell types: a major population displaying autofluorescence levels similar to those of cells from undifferentiated embryoid bodies and a second population displaying higher autofluorescence. RNA analyses demonstrated that the transcription of alpha-fetoprotein was associated only with the more highly autofluorescent population, indicating that flow cytometry provides a novel mechanism for the separation of undifferentiated cells from differentiated endoderm cells in F9 embryoid bodies.
Figures

References
-
- Adamson EA, Grover A. The production and maintenance of a functioning epithelial layer from embryonal carcinoma cells. In: Silver LM, Martin GR, Strickland S, editors. Teratocarcinoma Stem Cells. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1983. pp. 69–81.
-
- Aubin JE. Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem. 1979;27:36–43. - PubMed
-
- Becker S, Casanova J, Grabel LB. Localization of endoderm-specific mRNAs in differentiating F9 embryoid bodies. Mech Devel. 1992;37:3–12. - PubMed
-
- Benson RC, Meyer RA, Zaruba ME, McKhann GM. Cellular autofluorescence—is it due to flavins? J Histochem Cytochem. 1979;27:44–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
