Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen
- PMID: 8598466
- PMCID: PMC1950465
Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen
Abstract
Recombinant adenovirus (rAd), deleted of critical genes that enable viral replication and replaced with genes encoding heterologous proteins, has been shown to be a safe and effective vector in gene therapy studies. To evaluate a potential role for rAd as an immunogen, we used two different replication-defective type 2 rAds encoding the model Ag, beta-galactosidase (beta-gal). To determine whether rAd elicited the kind of immune responses therapeutic in an anti-tumor setting, the beta-gal-expressing adenocarcinoma, CT26.CL25, was used. Splenocytes from BALB/c mice immunized with 1 x 10(7) infectious units (iu) of rAd demonstrated anti-beta-gal activity after in vitro culture with the relevant L(d) beta-gal peptide. Adoptive transfer of these same splenocytes produced dramatic regression of established pulmonary metastases. However, when tumor-bearing mice were treated with 1 x 10(7) iu of rAd, no reduction in established disease was observed even when rAd was given with exogenous IL-2. To increase the viral dose delivered to each animal, we used an E1-E4-deleted rAd that could be grown to much higher titers. Significant reduction occurred with 10-fold more rAd (1 x10(8) iu) was administered. Exogenous IL-2 administration with 1 x 10(8) iu of rAd resulted in augmentation of this anti-tumor effect. These findings demonstrate that when using a nonreplicating virus, the viral dose is directly related to the immune response generated. These data constitute the first reported use of rAd in the treatment of an established experimental cancer and may have implication for the treatment of human cancer.
Figures


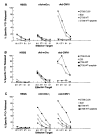

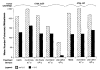

References
-
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. Preliminary report. N Engl J Med. 1988;319:1676. - PubMed
-
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159. - PubMed
-
- Alexander RB, Rosenberg SA. Long term survival of adoptively-transferred tumor infiltrating lymphocytes in mice. J Immunol. 1990;145:1615. - PubMed
-
- Boon T, Cerottini J, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognired by T lymphocytes. Annu Rev Immunol. 1994;12:337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
