Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases
- PMID: 8598468
- PMCID: PMC2040336
Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases
Abstract
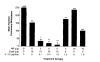
DNA immunization can result in the induction of Ag-specific cellular and humoral immune responses and in protective immunity in several Ag systems. To evaluate the utility of DNA-based immunization as a potential cancer treatment strategy, we employed an experimental murine tumor, CT26, expressing the model tumor-associated Ag, beta-galactosidase (beta-gal), designated CT26.CL25. A plasmid expressing beta-gal (pCMV/beta-gal) administered by particle-mediated gene delivery to the epidermis using a hand-held, helium-driven "gene gun" induced beta-gal-specific Ab and lytic responses. Immunization with this construct prevented the growth of pulmonary metastatic tumor, and the adoptive transfer of splenocytes generated by pCMV/beta-gal in vivo immunization and cultured in vitro with the beta-gal876-884 immunodominant peptide reduced the number of established pulmonary nodules. DNA immunization alone had little or no impact on the growth of established lung metastases. To enhance the function of DNA immunization for active immunotherapy, a panel of cytokines was added as adjuvants following DNA administration. Significant reduction in the number of established metastases was observed when human rIL-2, mouse rIL-6, human rIL-7, or mouse rIL-12 were given after DNA inoculation; mouse rIL-12 as an adjuvant had the most profound effect. These findings suggest that the cytokines involved in the activation and expansion of lymphocyte populations may improve the therapeutic effects of DNA immunization. Given the ease with which plasmid DNA can be prepared to high purity for safe use in humans with infectious diseases and cancers, DNA immunization administered together with cytokine adjuvant may be an attractive alternative to recombinant viral vaccines.
Figures


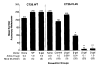

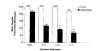
References
-
- Greenberg PD, Cheever MA, Fefer A. H-2 restriction of adoptive immunotherapy of advanced tumors. J Immunol. 1981;126:2100. - PubMed
-
- Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281. - PubMed
-
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwnrrzentruber D, Wei JP, White DE. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: preliminary report. N Engl J Med. 1988;319:1676. - PubMed
-
- Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264(716) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
