A lifetime-based fluorescence resonance energy transfer sensor for ammonia
- PMID: 8600839
- PMCID: PMC6905383
- DOI: 10.1006/abio.1995.9955
A lifetime-based fluorescence resonance energy transfer sensor for ammonia
Abstract
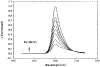
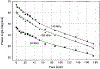
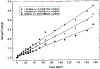
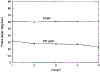
A lifetime-based optical NH3 sensor based on the principle of fluorescence resonance energy transfer was developed. The sensor consisted of sulforhodamine 101 as the donor, bromocresol green as the acceptor, ethyl cellulose as the polymer support, and tributyl phosphate as the plasticizer. When the concentration of NH3 changed, it caused a change in the decay time of the SR101, which was measured by phase-modulation fluorometry. At 100 MHz, increasing the concentration of NH3 from 0 to 175 ppm resulted in a decrease in phase angle of about 31 degrees and an increase in modulation of about 18%. Oxygen and carbon dioxide did not interfere with the sensor. However, a 30% relative humidity could cause a downward shift of the response by 5 degrees, while additional increase in the relative humidity to 100% showed little further effect. For a film thickness of 40 microns, the typical response and recovery times for 90% of total signal change were 1 and 2.5 min, respectively. The phase angle measurements for the same sample were reproducible for 5 days, with no special care of the film sample.
Figures



References
-
- Wolfbeis OS (Ed.) (1991) Fiber Optic Chemical Sensors and Biosensors, Vol. I and II, CRC Press, Boca Raton, FL.
-
- Lakowicz JR (Ed.) (1994) Topics in Fluorescence Spectroscopy, Vol. 4, Probe Design and Chemical Sensing, Plenum Press, New York.
-
- Seitz WR (1988) CRC Crit. Rev. Anal. Chem 19, 135–173.
-
- Rao G, Bambot SB, Kwong CW, Szmacinski H, Sipior J, Holavanahali R, and Carter G (1994) in Topics in Fluorescence Spectroscopy, Vol. 4, Probe Design and Chemical Sensing (La kowicz JR, Ed.), pp. 417–448, Plenum Press, New York.
-
- Uttamlal M, and Walt DR (1995) Biotechnology, 13, 597–601.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

