Crystal structure of the kinesin motor domain reveals a structural similarity to myosin
- PMID: 8606779
- PMCID: PMC2851642
- DOI: 10.1038/380550a0
Crystal structure of the kinesin motor domain reveals a structural similarity to myosin
Abstract
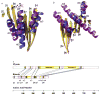
Kinesin is the founding member of a superfamily of microtubule based motor proteins that perform force-generating tasks such as organelle transport and chromosome segregation. It has two identical approximately 960-amino-acid chains containing an amino-terminal globular motor domain, a central alpha-helical region that enables dimer formation through a coiled-coil, and a carboxy-terminal tail domain that binds light chains and possibly an organelle receptor. The kinesin motor domain of approximately 340 amino acids, which can produce movement in vitro, is much smaller than that of myosin (approximately 850 amino acids) and dynein (1,000 amino acids), and is the smallest known molecular motor. Here, we report the crystal structure of the human kinesin motor domain with bound ADP determined to 1.8-A resolution by X-ray crystallography. The motor consists primarily of a single alpha/beta arrowhead-shaped domain with dimensions of 70 x 45 x 45 A. Unexpectedly, it has a striking structural similarity to the core of the catalytic domain of the actin-based motor myosin. Although kinesin and myosin have virtually no amino-acid sequence++ identity, and exhibit distinct enzymatic and motile properties, our results suggest that these two classes of mechanochemical enzymes evolved from a common ancestor and share a similar force-generating strategy.
Figures

Comment in
-
Motor proteins. A two-way structure.Nature. 1996 Apr 11;380(6574):483-4. doi: 10.1038/380483a0. Nature. 1996. PMID: 8606761 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

